the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Development of electrochemical sensors based on silver nanoparticles electrodeposited on gold screen-printed electrodes: application to nitrate trace analysis in water
Najib Ben Messaoud
Marília Barreiros dos Santos
Begoña Espiña
Raquel Barbosa Queirós
A simple, reusable and sensitive electrochemical sensor based on a gold screen-printed electrode modified with silver nanoparticles has been developed for the detection of nitrate in water. Scanning electron microscopy, square wave voltammetry and electrochemical impedance spectroscopy were used to characterize the modification of the electrode surface. The modified electrode with different silver nanoparticle loadings was also tested, as well as the influence of scan rate on the reduction of nitrate. The sensor exhibited a wide linear response to nitrate from 100 to 1500 µM and a detection limit of 7.7 µM, which is significantly less than the maximum contaminant level admitted in drinking water (800 µM). The reproducibility, repeatability and selectivity of the sensor have also been examined. The suitability of the proposed sensor for real sample detection was successfully demonstrated via recovery studies performed in spiked tap water samples. The proposed approach was used to determine nitrate in freshwater, and the results were in good agreement with those obtained from a commercial nitrate sensor. These advantages make the developed sensor a promising alternative approach for integration into an online monitoring system for water monitoring.
- Article
(2708 KB) - Full-text XML
- BibTeX
- EndNote
Nitrogen species play an important role in determining freshwater ecosystem health and ecological status. Nitrate (NO) can be found naturally in soil, water, vegetables and foods. Moreover, these ions are widely used as food preservatives and fertilizers; thus, wastewater from anthropogenic activities such as agriculture and industry results in significant hazards to environmental waters (Wakida and Lerner, 2005). High levels of nitrate can cause unnatural blooms of aquatic plants and algae, resulting in “red tides” and the death of fish (Camargo et al., 2005; Lee, 2006). In addition, when nitrate enters the food chain through groundwater or surface water, it can have negative effects on human health. In infants, for example, nitrate is the main cause of blue baby disease or methemoglobinemia, through drinking water containing high concentrations of nitrate (Knobeloch et al., 2000). Thus, concern has been expressed regarding high nitrate ion levels in water. For this reason, the maximum contaminant level of nitrate in drinking water should not exceed 800 µM according to recommendations of the World Health Organization (WHO; World Health Organization, 2006). Therefore, developing sensors for detecting nitrate at trace concentrations has become a very important subject of research. Several analytical methods have been reported and used for the determination of nitrate in different matrices including ion chromatography (Lopez-Moreno et al., 2016), high-performance liquid chromatography (HPLC) (Kodamatani et al., 2009), ultra-performance liquid chromatography (UPLC) (Siddiqui et al., 2015), UV spectrometry (Alahi and Mukhopadhyay, 2018) and mass spectroscopy (Li et al., 2011). However, these methods require dedicated equipment, large bench space, long analysis times and highly qualified technicians which makes them expensive and inconvenient for faster detection of nitrate. Currently, much interest is given to developing rapid, accurate and portable methods for the determination of nitrate in the field, and electrochemical sensors are one of the most promising approaches. In the scenario of electroanalytical methods dedicated to automated determination of large numbers of samples, an excited and intelligent strategy is the coupling of a flow injection analysis (FIA) system with an electrochemical sensors for the detection of nitrate (Gamboa et al., 2009b; Badea et al., 2001; Bui et al., 2016). FIA offers interesting advantages such as versatility, accuracy, low cost, speed and automation, among others. Modification of the surfaces of such electrochemical sensors with nanomaterials can enhance these advantages due to their high surface area, high electrical conductivity, high catalytic activity and strong adsorption capability, which have made them very valuable (Ahmad et al., 2017; Ben Messaoud et al., 2018, 2017b; Ben Jaballah et al., 2022, 2021; Gaur et al., 2021; Paulraj et al., 2021, 2020; Lokesh et al., 2016; Thanigai Arul et al., 2016; Saasa et al., 2015). Some of the nanomaterials which have been used as catalysts for the electro-reduction of nitrate are platinum (Dima et al., 2003), copper (Motaghedifard et al., 2021), carbon (Wang et al., 2018), gold (Zhao et al., 2019) and silver (Wang et al., 2021a). Because of their excellent electrocatalytic activity and large surface-to-volume ratio, silver nanoparticles (AgNPs) have also been used for the modification of electrodes. However, the preparation and modification of electrochemical sensors based on AgNPs for nitrate in reported studies seem to be complex and time-consuming, such as the following composites containing AgNPs: AgNPs–chitosan–polyvinylpyrrolidone (Wang et al., 2021b), AgNPs–polypyrrole (Atmeh and Alcock-Earley, 2011; Ghanbari, 2013) and AgNPs–polymethacrylic acid (Bonyani et al., 2016). Although the reported levels of nitrate are low, it is still a challenge to determine nitrate directly in water samples without the need for pH adjustment (Patella et al., 2021; Hanane et al., 2020).
In the present work, a simple, reusable and easy-to-prepare electrochemical sensor based on a gold screen-printed electrode (AuSPE) modified with AgNPs was developed. In order to obtain the highest sensing performance, the optimal electrodeposition time of AgNPs, as well as the best deposition technique, was evaluated. Furthermore, a NaCl solution, at neutral pH, was used as electrolyte, providing the opportunity to directly measure nitrate content in real water samples without the need to change the electrolyte pH, condition which is often required for the detection of nitrate as reported in the literature (Liang et al., 2016; Li et al., 2012; Tsai et al., 2010). The surface modification was characterized by scanning electron microscopy (SEM), electrochemical impedance spectroscopy (EIS) and square wave voltammetry (SWV) as well as the analytical performance of the modified electrodes. The sensitivity, reproducibility, selectivity, repeatability and stability of the sensor have been determined. The developed analytical approach was applied successfully for the determination of nitrate in real environmental freshwater samples without the need for pH adjustment, and equivalent results were obtained and compared to commercial nitrate sensors. Moreover, the developed nitrate electrochemical sensor was applied to real environmental freshwater samples without spiking with standard solutions.
The features of the proposed sensor, including simplicity and celerity of the fabrication and analysis, as well as the good performance and stability in time at room temperature, make this sensing technology a promising alternative approach for its integration into portable sensing platforms for nitrate detection in water.
2.1 Reagents and materials
Potassium nitrate (KNO3), silver nitrate (AgNO3), sodium chloride (NaCl), potassium ferricyanide [K3Fe(CN)6], potassium ferrocyanide [K4Fe(CN)6], interfering substances (calcium dichloride (CaCl2), potassium chloride (KCl), sodium nitrite (NaNO2), sodium bicarbonate (NaHCO3), sodium acetate (C2H3NaO2) magnesium sulfate (MgSO4)), and a phosphate-buffered saline (PBS) tablet were purchased from Sigma-Aldrich. Gold screen-printed electrodes (AuSPEs) (ref. C223AT) and the respective connector (ref. DSC) were purchased from DropSens, Spain. The AuSPE consists of a ceramic substrate with a three-electrode system where the gold and counter electrodes are made of gold, and the reference electrode is made of silver. The diameter of the working electrode was 1.6 mm, resulting in a geometric area of 2.01 mm2.
2.2 Solutions
A stock solution of nitrate and of the interferents were dissolved in NaCl solution (0.6 M). A 10 mM sulfuric acid solution (H2SO4) was used to clean the electrode surface before the modification. A 3.5 mM AgNO3 solution dissolved in KNO3 was used as electrolyte solution (at a concentration of 100 mM). A 5 mM [Fe(CN)6] solution was prepared in 10 mM PBS solution (pH = 7.4) using K3Fe(CN)6 and K4Fe(CN)6. Freshwater samples were filtered through a 0.2 µm sterile polyethersulfone filter and tested with the developed electrochemical sensors as well as with a commercial nitrate potentiometric electrode (perfectION™, ref. 51344727, from Mettler Toledo).
2.3 Instrumentation and electrochemical measurements
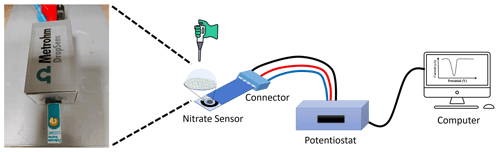
The electrochemical measurements were performed using a potentiostat/galvanostat (PGSTAT302N/FRA32M) monitored by NOVA 2.1 software from Metrohm Autolab, the Netherlands. The sensors were connected to the potentiostat using a connector to read out the SPEs (ref. DSC, DropSens, Spain). The scheme of the experimental setup is presented in Scheme 1.
For electrodeposition of the AgNPs onto the gold electrodes surface, chronoamperometry was used, applying a −0.2 V potential step for 7 s.
Cyclic voltammetry (CV) measurements were recorded over a potential range from 0 to +1.5 V in 0.01 M H2SO4 at a scan rate 100 mV s−1 for the pretreatment of the AuSPE and from −1.4 to 0 V for the study of the electrochemical behavior of the nitrate sensor using different scan rates between 10 and 100 mV s−1 and 5 mV as potential step.
To confirm the electrode surface modification, EIS measurements were carried out in the frequency range from 100 kHz to 0.1 Hz and with a root mean square (rms) perturbation voltage of 5 mV in a 5 mM of [Fe(CN)6] solution.
To characterize the electrode modification and test the nitrate sensor, SWV measurements were carried out at a pulse amplitude of 60 mV with a frequency of 100 Hz. The potential varied from 0.0 to −1.4 V.
For the testing of the analytical performance of the sensors, different concentrations of nitrate were tested (from 50 to 10 000 µM).
Potentiometric measurements of real samples were performed at room temperature using a commercial nitrate ion-selective electrode (ISE) of inner filling solution (perfectION™, ref. 51344727) connected to a pH/ion meter S220 (SevenCompact™, ref. 30019028) from Mettler Toledo.
2.4 Electrode modification
The AuSPE was rinsed with isopropanol and Milli-Q water and dried with N2. Secondly, The AuSPE was conditioned in sulfuric acid solution (H2SO4, 0.01 M) by cyclic voltammetry between E1 = 0 V and E2 = 1.5 V at 100 mV s−1 until reproducible cycles were obtained (20 cycles). The electrodeposition process of the AgNPs onto the gold electrode surface was previously reported (Ivanišević, 2023; Zahran et al., 2021) for electrochemical sensors development. Briefly, the electrodeposition of the AgNPs onto the gold electrodes was performed by casting a drop (75 µL) of KNO3 solution containing 3.5 mM of AgNO3 on the surface of the AuSPE, followed by the application of a voltage (E = −0.2 V), using a chronoamperometry method for 7 s. Thereafter, the modified electrode was washed with Milli-Q water and dried with N2.
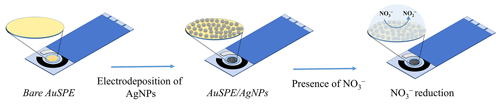
Figure 1Schematic illustration of the electrode modification process of the proposed nitrate sensor.
Figure 1 shows the fabrication process of the proposed reusable and easy-to-fabricate nitrate sensor. According to the references (Huang et al., 2012; Dhanya et al., 2013), the most probable mechanism is that a AgNP film catalytically reduces H+ to form nascent hydrogen. This produced nascent hydrogen will then react with nitrate, which will further react with the electrode surface to form nitrite. Therefore, AgNP layers should lead to the formation of a catalyst that can be used for the selective electrochemical reduction of NO to NO according to the following reaction:
3.1 SEM characterization
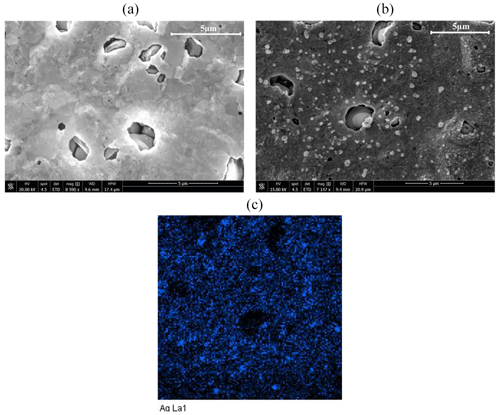
The electrode surfaces before and after modification were investigated by SEM (Fig. 2). Figure 2a shows the SEM image of the bare AuSPE. After electrode surface modification, the SEM image (Fig. 2b) clearly shows the presence of AgNPs. To confirm that, energy-dispersive X-ray mapping (Fig. 2c) reported the presence and the homogeneous distribution of AgNPs (blue color) on the electrode surface.
3.2 Optimization of the modified electrode preparation
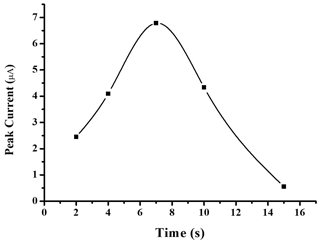
In order to control the exact quantity of silver nanoparticles deposited on the gold surface, the electrodeposition realized to form AuSPE/AgNPs electrodes was controlled by time. Therefore, the effect of electrodeposition time was studied by varying the time range from 5 to 15 s. As shown in Fig. 3, the highest current response of the proposed sensor for nitrate detection was achieved with an electrodeposition of 7 s. Thus, the electrodeposition time of 7 s was selected and used for further studies to maximize the sensitivity of the sensor for the determination of nitrate.
3.3 Electrochemical properties of the modified electrode
Unmodified and modified electrodes were characterized by SWV in the presence of nitrate. Figure 4 shows SWV in NaCl solution (0.6 M) containing 10mM nitrate at bare AuSPE and AuSPE/AgNPs. After modifying the electrode with AgNPs, a reduction peak of nitrate was observed at −1.05 V, indicating that their presence on the surface of AuSPE catalyzes the reduction of nitrate. This result could be attributed to the unique physicochemical and catalytic properties of AgNPs, as well as their superior electrical conductivity (Paul et al., 2021; Zahran et al., 2021).
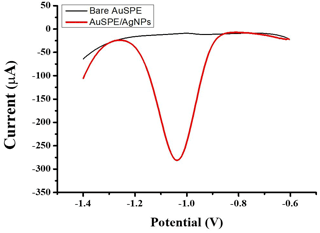
Figure 4SWV of bare AuSPE and AuSPE/AgNPs in NaCl solution (0.6 M, pH = 7) containing 10 mM nitrate.
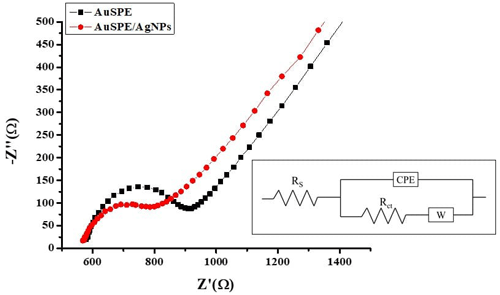
The EIS method was used to investigate the electron transfer on the interface of the bare and modified AuSPEs. This technique has been widely employed for the electrochemical behavior investigation of the reaction dynamics and the membrane/solution structure (Ben Messaoud et al., 2016, 2017a, 2023, 2022). Figure 5 presents Nyquist plots of 5 mM [Fe(CN)6] at different electrodes. The Nyquist spectra of bare AuSPE and AuSPE/AgNPs were fitted using an appropriate equivalent circuit shown in the inset of Fig. 5. The circuit comprises the solution resistance (Rs) in series with a parallel combination of a charge transfer resistance (Rct) and Warburg impedance (W); both are in parallel with constant phase element (CPE). A decrease of Rct was observed when the AuSPE was modified with AgNPs from 335.6 to 290.1 Ω, indicating that AgNPs enhance the conductivity and facilitate electron transfer compared with the bare AuSPE.
3.4 Electrochemical behavior of nitrate at the modified electrode
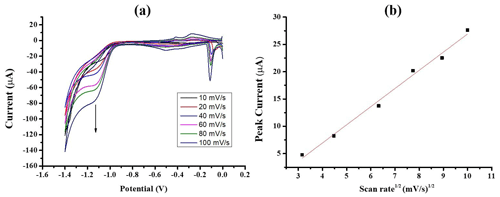
Useful information involving electrochemical mechanisms generally can be acquired from the relationship between current density and scan rate. Therefore, the influence of scan rate on the reduction of 10 mM nitrate at AuSPE/AgNPs was studied by CV and is illustrated in Fig. 6a. It can be seen that peak current density increases linearly with the square root of scan rate in the range from 10 to 100 mV s−1 (Fig. 6b), indicating that the reduction of nitrate was controlled by diffusion processes in the range of scan rate studied, which is coherent well with AgNPs-based sensors for nitrate reported in the literature (Wang et al., 2021b; Bonyani et al., 2016; Ghanbari, 2013).
3.5 Determination of nitrate
3.5.1 Analytical performance
The analytical performance of the sensor was studied and optimized in order to find a stable relation between the variation of NO3 concentration and the measured current. The determination of nitrate using the optimized configuration, AuSPE/AgNPs, was performed by SWV for different nitrate concentrations and is shown in Fig. 7a. With the increase in nitrate concentration, more nitrate ions will be available on the electrode surface for reduction, and there is a proportional increase in the measured peak current. The calibration plot constructed from the SWV response, illustrated in Fig. 7b, shows a linear range from 100 to 1500 µM. The detection limit was determined to be 7.7 µM (N = 4), which was calculated as (3 × SDblank) slope (Shrivastava and Gupta, 2011).
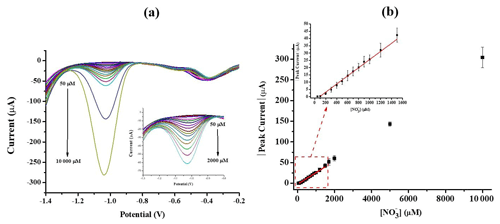
Figure 7(a) SWV of different concentrations of nitrate from 50 to 10 000 µM at AuSPE/AgNPs in NaCl (0.6 M, pH = 7); the inset is the magnified SWV plot at low concentration. (b) Corresponding calibration curve (N = 4).
Table 1Comparison of the analytical performance of the proposed sensor for nitrate detection with literature reports.
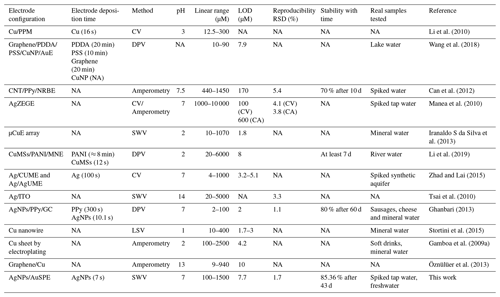
LOD: limit of detection; CV: cyclic voltammetry; DPV: differential pulse voltammetry; Cu: copper; PPM: plated platinum microelectrode; CuNP: copper nanoparticles; AuE: gold electrode; PDDA: poly(diallyldiamine chloride); PSS: poly(sodium 4-styrene sulfonate); CNT: carbon nanotube; PPy: polypyrrole; NRBE: nitrate reductase biofilm electrode; AgZEGE: Ag-doped zeolite-expanded graphite-epoxy composite electrode; µCuE: copper microelectrodes; CuMSs: copper microspheres; PANI: polyaniline; MNE: micro-needle electrode; GC: glassy carbon; Ag: silver; CUME: carbon ultramicroelectrode; AgUME: silver ultramicroelectrode; ITO: indium tin oxide; AgNPs: silver nanoparticles. LSV: linear sweep voltammetry; NA: not available.
The performance obtained by the proposed sensor is important as it is clear from the comparison with previous reports on modified electrode sensors with nanomaterials, shown in Table 1. Also, remarkable advantages such as simplicity and fast preparation time (of only 7 s) were achieved compared to other reported architectures from the literature, as shown in Table 1. Another major merit of the current approach is that the developed sensor can be used around neutral pH, which is a necessary condition for direct nitrate analysis in real environmental samples. Most nitrate sensors based on other modified materials can only be used effectively in acidic or basic aqueous conditions (Liang et al., 2016; Li et al., 2012; Tsai et al., 2010), which does not allow them to directly monitor nitrate levels in water that has a pH close to neutral in nature.
3.5.2 Reproducibility, repeatability and stability at AuSPE/AgNPs
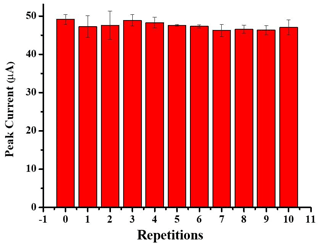
Reproducibility, repeatability and stability are key elements of the sensor performance. Reproducibility is the ability of a sensor to yield identical results regardless of the number of times the sensor fabrication is repeated. The reproducibility of the fabricated sensor towards nitrate detection was calculated by taking the response of four separately fabricated sensors (N = 4). For that, the relative standard deviation (RSD) value of the current values measured from each fabricated sensor was calculated. RSD less than 10 % is desirable. The RSD for measuring 2000 µM nitrate by SWV at four different electrodes prepared in the same way was 1.7 %. This shows that the AuSPE/AgNPs modification has a good reproducibility, and it is better than that achieved by some reports (Can et al., 2012; Manea et al., 2010; Li et al., 2019; Andreoli et al., 2011; Zhou et al., 2013; Tsai et al., 2010). Since there is no significant difference between the prepared electrodes, the same calibration curve could be applied to all the sensors. The repeatability of the single AuSPE/AgNP-modified sensor toward 2 mM nitrate was studied by observing the current response over 11 independent SWV measurements under the same experimental conditions. Between measurements, the electrodes were rinsed with Milli-Q water and dried with N2. An RSD of 2 % (Fig. 8) was achieved for the repeatability tests, which is superior to other reported sensors (Ahmad et al., 2017; Zhou et al., 2013; Alagha et al., 2020). The stability of the proposed sensor was also investigated by measuring the electrode response with 1 mM nitrate on different days. Between measurements, the electrodes were stored dry and at room temperature, since it was envisioned that the application of these sensors is in a real scenario where sensors can only be kept at room temperature. The current response decreased to 91.3 % after 6 d, while the current response retained 85.3 % of the initial response after 43 d, which indicates the good storage capability of the present sensor. The stability obtained by the present sensor is better compared to that obtained in Zhou et al. (2013) (92 % in 30 d) and Can et al. (2012) (70 % in 10 d), but in those studies the electrode was kept at 4 °C. These studies suggest that storage at lower temperature could increase the stability of the sensor for longer periods of time (> 45 d). Therefore, other conditions must be tested in the future to increase the stability of our developed sensor.
3.5.3 Interferences and practical application
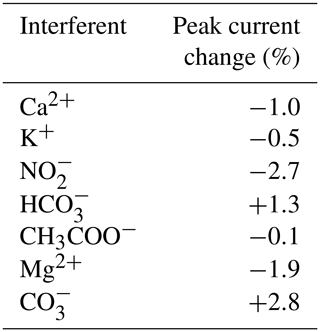
The selectivity of the electrochemical sensor towards nitrate was investigated in the presence of some possible interfering substances at concentrations 10-fold higher than nitrate. The interferents tested included Ca2+, K+, NO, HCO, CH3COO−, Mg2+ and CO. As can be seen in Table 2, none of them exhibited a significant change in the nitrate response; the signal change was less than 3 % for all interferents. The proposed sensor thus demonstrated a good selectivity for the determination of nitrate.
In order to evaluate the performance of AuSPE/AgNPs in practical analytical applications, the sensor performance for the determination of nitrate in real water samples was carried out. To keep the same conditions performed for the calibration curve, a solution with the same ionic-strength properties was used for the measurement, by mixing equal parts of water sample and a 1.2 M NaCl solution. The doped samples were then spiked with different amounts of nitrate (500, 1000 and 5000 µM). Accuracy was determined through recovery experiments. The recovery percentage (%) was calculated by the following equation according to IUPAC Recommendations 2002 (Burns et al., 2002).
where [NO]found is the concentration of nitrate measured from the spiked sample, [NO]added is the concentration of nitrate added (spike value) and [NO]sample is the concentration of nitrate from the original sample which in this case is equal to zero because nitrate was not found in the tap water samples. Recovery values close to 100 % indicate higher trueness of the method (Taverniers et al., 2004). The recoveries achieved were 101.9 %, 103.2 %, and 101.0 %, respectively, indicating that the sensor is adequate for application in water samples.
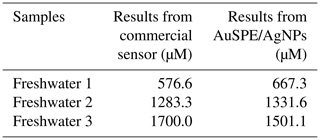
To validate the potential application of the developed sensor in natural water samples, three freshwater samples were tested by using the proposed sensor and comparing with a commercial potentiometric ion-selective nitrate sensor (Table 3). Comparable results were obtained using both sensors, with an RSD between 2.6 % and 10.3 % for three samples tested, indicating the trustful analytical performances of the developed sensor.
A simple, reusable and sensitive electrochemical sensor based on a AuSPE modified with AgNPs has been developed for nitrate detection. Different amounts of AgNPs were tested, and the best experimental condition for higher sensitivity was obtained with a electrodeposition time of 7s. The proposed AgNP-based sensor shows important features such as a low detection limit of 7.7 µM and wide linear detection range from 100 to 1500 µM. Moreover, the sensor showed a good repeatability and stability, a short time of analysis, and a high selectivity towards nitrate compared to other possible interferent species (Ca2+, K+, NO, HCO, CH3COO−, Mg2+ and CO). The practical application in spiked tap water samples was successfully carried out, achieving good recoveries (between 101.0 % and 103.2 %). Moreover, the developed sensor was efficaciously applied for the determination of nitrate in freshwater samples with comparable results to commercial nitrate sensors.
The unique advantages of the proposed sensor, such as small footprint, cost-effectiveness, simplicity, fast analysis, low sample volumes (typically in the microliter range) required and good performance provide a promising alternative approach for the integration of the developed sensor into a portable or automated sensing platform for nitrate detection in water. In this way, the sensing system may be helpful for screening of nitrate in water bodies, speeding up decision-making at different levels.
No data sets were used in this article.
NBM: investigation, methodology, formal analysis, validation, writing (original draft and review and editing). MBdS: conceptualization, investigation, methodology, formal analysis, writing ( original draft and review and editing). BE: conceptualization, supervision, writing (review and editing), funding acquisition, project management. RBQ: conceptualization, investigation, methodology, formal analysis, funding acquisition, project management, supervision, writing (original draft and review and editing).
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Sensors and Measurement Science International SMSI 2023”. It is a result of the 2023 Sensor and Measurement Science International (SMSI) Conference, Nuremberg, Germany, 8–11 May 2023.
This research has been supported by the EEA (European Economic Area) and Norway Grants 2014–2021 through the funded project OPTIRAS (grant no. EEA.BG.Call4.023.2020) and by the following programs: POCI – PROGRAMA OPERACIONAL COMPETITIVIDADE E INTERNACIONALIZAÇÃO, Co-promotion – COMPETE under the Portugal 2020 Framework through the RWClean project (grant no. POCI-01-0247-FEDER-070109).
This paper was edited by Rosario Morello and reviewed by three anonymous referees.
Ahmad, R., Bhat, K. S., Ahn, M.-S., and Hahn, Y.-B.: Fabrication of a robust and highly sensitive nitrate biosensor based on directly grown zinc oxide nanorods on a silver electrode, New J. Chem., 41, 10992–10997, https://doi.org/10.1039/C7NJ02526B, 2017.
Alagha, O., Manzar, M. S., Zubair, M., Anil, I., Mu'azu, N. D., and Qureshi, A.: Comparative adsorptive removal of phosphate and nitrate from wastewater using biochar-MgAl LDH nanocomposites: coexisting anions effect and mechanistic studies, Nanomaterials, 10, 336, https://doi.org/10.3390/nano10020336, 2020.
Alahi, M. E. E. and Mukhopadhyay, S. C.: Detection methods of nitrate in water: A review, Sensor. Actuat. A-Phys., 280, 210–221, https://doi.org/10.1016/j.sna.2018.07.026, 2018.
Andreoli, E., Annibaldi, V., Rooney, D. A., Liao, K.-S., Alley, N. J., Curran, S. A., and Breslin, C. B.: Electrochemical conversion of copper-based hierarchical micro/nanostructures to copper metal nanoparticles and their testing in nitrate sensing, Electroanalysis, 23, 2164–2173, https://doi.org/10.1002/elan.201100105, 2011.
Atmeh, M. and Alcock-Earley, B. E.: A conducting polymer/Ag nanoparticle composite as a nitrate sensor, J. Appl. Electrochem., 41, 1341–1347, https://doi.org/10.1007/s10800-011-0354-4, 2011.
Badea, M., Amine, A., Palleschi, G., Moscone, D., Volpe, G., and Curulli, A.: New electrochemical sensors for detection of nitrites and nitrates, J. Electroanal. Chem., 509, 66–72, https://doi.org/10.1016/S0022-0728(01)00358-8, 2001.
Ben Jaballah, M., Ben Messaoud, N., and Dridi, C.: Nanoengineering of new cost-effective nanosensor based on functionalized MWCNT and Ag nanoparticles for sensitive detection of BPA in drinking water, Appl. Phys. A, 127, 713, https://doi.org/10.1007/s00339-021-04857-3, 2021.
Ben Jaballah, M., Ben Messaoud, N., and Dridi, C.: Development of cost-effective and sustainable sensing nanoplatform based on green AgNPs for the determination of BPA in water, J. Mater. Sci.-Mater. El., 33, 6981–6998, https://doi.org/10.1007/s10854-022-07877-8, 2022.
Ben Messaoud, N., Braik, M., Dridi, C., Ben Ali, M., Ali, A., Abbas, M. N., and Errachid, A.: Optical, electrical and perchlorate sensing properties of a new CoPc derivative, Sens. Lett., 14, 928–937, https://doi.org/10.1166/sl.2016.3713, 2016.
Ben Messaoud, N., Baraket, A., Dridi, C., Nooredeen, N. M., Abbas, M. N., and Errachid, A.: A highly sensitive miniaturized impedimetric perchlorate chemical sensor, IEEE Sens. J., 18, 1343–1350, https://doi.org/10.1109/JSEN.2017.2780445, 2017a.
Ben Messaoud, N., Ghica, M. E., Dridi, C., Ali, M. B., and Brett, C. M.: Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A, Sensor. Actuat. B-Chem., 253, 513–522, https://doi.org/10.1016/j.snb.2017.06.160, 2017b.
Ben Messaoud, N., Lahcen, A. A., Dridi, C., and Amine, A.: Ultrasound assisted magnetic imprinted polymer combined sensor based on carbon black and gold nanoparticles for selective and sensitive electrochemical detection of Bisphenol A, Sensor. Actuat. B-Chem., 276, 304–312, https://doi.org/10.1016/j.snb.2018.08.092, 2018.
Ben Messaoud, N., Dos Santos, M. B., Vieira, A., Garrido-Maestu, A., Espiña, B., and Queirós, R. B.: A novel portable label-free electrochemical immunosensor for ultrasensitive detection of Aeromonas salmonicida in aquaculture seawater, Anal. Bioanal. Chem., 414, 6591–6600, https://doi.org/10.1007/s00216-022-04219-9, 2022.
Ben Messaoud, N., Dos Santos, M. B., Trocado, V., Nogueira-Silva, C., and Queirós, R.: A novel label-free electrochemical immunosensor for detection of surfactant protein B in amniotic fluid, Talanta, 251, 123744, https://doi.org/10.1016/j.talanta.2022.123744, 2023.
Bonyani, M., Mirzaei, A., Leonardi, S. G., and Neri, G.: Silver nanoparticles/polymethacrylic acid (AgNPs/PMA) hybrid nanocomposites-modified electrodes for the electrochemical detection of nitrate ions, Measurement, 84, 83–90, https://doi.org/10.1016/j.measurement.2016.02.005, 2016.
Bui, M.-P. N., Brockgreitens, J., Ahmed, S., and Abbas, A.: Dual detection of nitrate and mercury in water using disposable electrochemical sensors, Biosens. Bioelectron., 85, 280–286, https://doi.org/10.1016/j.bios.2016.05.017, 2016.
Burns, D. T., Danzer, K., and Townshend, A.: Use of the term “recovery” and “apparent recovery” in analytical procedures (IUPAC Recommendations 2002), Pure Appl. Chem., 74, 2201–2205, https://doi.org/10.1351/pac200274112201, 2002.
Camargo, J. A., Alonso, A., and Salamanca, A.: Nitrate toxicity to aquatic animals: a review with new data for freshwater invertebrates, Chemosphere, 58, 1255–1267, https://doi.org/10.1016/j.chemosphere.2004.10.044, 2005.
Can, F., Ozoner, S. K., Ergenekon, P., and Erhan, E.: Amperometric nitrate biosensor based on Carbon nanotube/Polypyrrole/Nitrate reductase biofilm electrode, Mater. Sci. Eng. C, 32, 18–23, https://doi.org/10.1016/j.msec.2011.09.004, 2012.
Dhanya, S., Saumya, V., and Rao, T. P.: Synthesis of silver nanoclusters, characterization and application to trace level sensing of nitrate in aqueous media, Electrochim. Acta, 102, 299–305, https://doi.org/10.1016/j.electacta.2013.04.017, 2013.
Dima, G. E., De Vooys, A. C. A., and Koper, M. T. M.: Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions, J. Electroanal. Chem., 554, 15–23, https://doi.org/10.1016/S0022-0728(02)01443-2, 2003.
Gamboa, J. C., Pena, R. C., Paixão, T. R., and Bertotti, M.: A renewable copper electrode as an amperometric flow detector for nitrate determination in mineral water and soft drink samples, Talanta, 80, 581–585, https://doi.org/10.1016/j.talanta.2009.07.028, 2009a.
Gamboa, J. C. M., Peña, R. C., Paixão, T. R. L. C., and Bertotti, M.: A renewable copper electrode as an amperometric flow detector for nitrate determination in mineral water and soft drink samples, Talanta, 80, 581–585, https://doi.org/10.1016/j.talanta.2009.07.028, 2009b.
Gaur, R., Padhy, H. M., and Elayaperumal, M.: Surface plasmon assisted toxic chemical NO2 gas sensor by Au ZnO functional thin films, J. Sens. Sens. Syst., 10, 163–169, https://doi.org/10.5194/jsss-10-163-2021, 2021.
Ghanbari, K.: Silver nanoparticles dispersed in polypyrrole matrixes coated on glassy carbon electrode as a nitrate sensor, Anal. Bioanal. Electrochem., 5, 46–58, 2013.
Hanane, K., Messaoud, B., Houcine, B., and Moncef, T.: Highly sensitive modified glassy carbon sensor based on TDAN for nitrate detection in real water, Monatsh. Chem., 151, 153–158, https://doi.org/10.1007/s00706-019-02547-8, 2020.
Huang, M., Henry, J. B., Berkes, B. B., Maljusch, A., Schuhmann, W., and Bondarenko, A. S.: Towards a detailed in situ characterization of non-stationary electrocatalytic systems, Analyst, 137, 631–640, https://doi.org/10.1039/C1AN15671C, 2012.
Iranaldo S da Silva, de Araujo, W. R., Paixão, T. R., and Angnes, L.: Direct nitrate sensing in water using an array of copper-microelectrodes from flat flexible cables, Sensor. Actuat. B-Chem., 188, 94–98, https://doi.org/10.1016/j.snb.2013.06.094, 2013.
Ivanišević, I.: The Role of Silver Nanoparticles in Electrochemical Sensors for Aquatic Environmental Analysis, Sensors, 23, 3692, https://doi.org/10.3390/s23073692, 2023.
Knobeloch, L., Salna, B., Hogan, A., Postle, J., and Anderson, H.: Blue babies and nitrate-contaminated well water, Environ. Health Perspect., 108, 675–678, https://doi.org/10.1289/ehp.00108675, 2000.
Kodamatani, H., Yamazaki, S., Saito, K., Tomiyasu, T., and Komatsu, Y.: Selective determination method for measurement of nitrite and nitrate in water samples using high-performance liquid chromatography with post-column photochemical reaction and chemiluminescence detection, J. Chromatogr. A, 1216, 3163–3167, https://doi.org/10.1016/j.chroma.2009.01.096, 2009.
Lee, Y. S.: Factors affecting outbreaks of high-density Cochlodinium polykrikoides red tides in the coastal seawaters around Yeosu and Tongyeong, Korea, Mar. Pollut. Bull., 52, 1249–1259, https://doi.org/10.1016/j.marpolbul.2006.02.024, 2006.
Li, Y., Sun, J., Bian, C., Tong, J., and Xia, S.: Electrodeposition of copper nano-clusters at a platinum microelectrode for trace nitrate determination, Procedia Engineer., 5, 339–342, https://doi.org/10.1016/j.proeng.2010.09.117, 2010.
Li, Y., Whitaker, J. S., and McCarty, C. L.: Reversed-phase liquid chromatography/electrospray ionization/mass spectrometry with isotope dilution for the analysis of nitrate and nitrite in water, J. Chromatogr. A, 1218, 476–483, https://doi.org/10.1016/j.chroma.2010.11.073, 2011.
Li, Y., Sun, J., Bian, C., Tong, J., and Xia, S.: Micro electrochemical sensor with copper nanoclusters for nitrate determination in freshwaters, Micro Nano Lett., 7, 1197–1201, https://doi.org/10.1049/mnl.2012.0533, 2012.
Li, Y., Han, H., Pan, D., and Zhang, P.: Fabrication of a micro-needle sensor based on copper microspheres and polyaniline film for nitrate determination in coastal river waters, J. Electrochem. Soc., 166, B1038, https://doi.org/10.1149/2.1281912jes, 2019.
Liang, J., Zheng, Y., and Liu, Z.: Nanowire-based Cu electrode as electrochemical sensor for detection of nitrate in water, Sensor. Actuat. B-Chem., 232, 336–344, https://doi.org/10.1016/j.snb.2016.03.145, 2016.
Lokesh, K., Kavitha, G., Manikandan, E., Mani, G. K., Kaviyarasu, K., Rayappan, J. B. B., Ladchumananandasivam, R., Sundeep Aanand, J., Jayachandran, M., and Maaza, M.: Effective Ammonia Detection Using n-ZnO/p-NiO Heterostructured Nanofibers, IEEE Sens. J., 16, 2477–2483, https://doi.org/10.1109/JSEN.2016.2517085, 2016.
Lopez-Moreno, C., Perez, I. V., and Urbano, A. M.: Development and validation of an ionic chromatography method for the determination of nitrate, nitrite and chloride in meat, Food Chem., 194, 687–694, https://doi.org/10.1016/j.foodchem.2015.08.017, 2016.
Manea, F., Remes, A., Radovan, C., Pode, R., Picken, S., and Schoonman, J.: Simultaneous electrochemical determination of nitrate and nitrite in aqueous solution using Ag-doped zeolite-expanded graphite-epoxy electrode, Talanta, 83, 66–71, https://doi.org/10.1016/j.talanta.2010.08.042, 2010.
Motaghedifard, M. H., Pourmortazavi, S. M., Alibolandi, M., and Mirsadeghi, S.: Au-modified organic/inorganic MWCNT/Cu/PANI hybrid nanocomposite electrode for electrochemical determination of nitrate ions, Microchim. Acta, 188, 1–12, https://doi.org/10.1007/s00604-021-04754-9, 2021.
Öznülüer, T., Özdurak, B., and Doğan, H. Ö.: Electrochemical reduction of nitrate on graphene modified copper electrodes in alkaline media, J. Electroanal. Chem., 699, 1–5, https://doi.org/10.1016/j.jelechem.2013.04.001, 2013.
Patella, B., Russo, R. R., O'Riordan, A., Aiello, G., Sunseri, C., and Inguanta, R.: Copper nanowire array as highly selective electrochemical sensor of nitrate ions in water, Talanta, 221, 121643, https://doi.org/10.1016/j.talanta.2020.121643, 2021.
Paul, D., Sachan, D., and Das, G.: Silver nanoparticles embedded on in-vitro biomineralized vaterite: A highly efficient catalyst with enhanced catalytic activity towards 4-Nitrophenol reduction, Molecular Catalysis, 504, 111433, https://doi.org/10.1016/j.mcat.2021.111433, 2021.
Paulraj, P., Umar, A., Rajendran, K., Manikandan, A., Kumar, R., Manikandan, E., Pandian, K., Mahnashi, M. H., Alsaiari, M. A., Ibrahim, A. A., Bouropoulos, N., and Baskoutas, S.: Solid-state synthesis of Ag-doped PANI nanocomposites for their end-use as an electrochemical sensor for hydrogen peroxide and dopamine, Electrochim. Acta, 363, 137158, https://doi.org/10.1016/j.electacta.2020.137158, 2020.
Paulraj, P., Umar, A., Rajendran, K., Manikandan, A., Sathamraja, A., Kumar, R., Manikandan, E., Pandian, K., Baskoutas, S., Algadi, H., Ibrahim, A. A., and Alsaiari, M. A.: Methylene blue intercalated layered MnO2 nanosheets for high-sensitive non-enzymatic ascorbic acid sensor, J. Mater. Sci.-Mater. El., 32, 8317–8329, https://doi.org/10.1007/s10854-021-05391-x, 2021.
Saasa, V., Mokwena, M., Dhonge, B., Manikandan, E., Kennedy, J., Murmu, P. P., Dewar, J., Erasmus, R., Whaley, M. F., and Mukwevho, E.: Optical and structural properties of multi-wall-carbon-nanotube-modified ZnO synthesized at varying substrate temperatures for highly efficient light sensing devices, Sensors & Transducers, 195, 9–17, http://hdl.handle.net/10204/8568 (last access: 29 May 2024), 2015.
Shrivastava, A. and Gupta, V. B.: Methods for the determination of limit of detection and limit of quantitation of the analytical methods, Chron. Young. Sci., 2, 21–25, https://doi.org/10.4103/2229-5186.79345, 2011.
Siddiqui, M. R., Wabaidur, S. M., ALOthman, Z. A., and Rafiquee, M. Z. A.: Rapid and sensitive method for analysis of nitrate in meat samples using ultra performance liquid chromatography–mass spectrometry, Spectrochim. Acta A, 151, 861–866, https://doi.org/10.1016/j.saa.2015.07.028, 2015.
Stortini, A. M., Moretto, L. M., Mardegan, A., Ongaro, M., and Ugo, P.: Arrays of copper nanowire electrodes: Preparation, characterization and application as nitrate sensor, Sensor. Actuat. B-Chem., 207, 186–192, https://doi.org/10.1016/j.snb.2014.09.109, 2015.
Taverniers, I., De Loose, M., and Van Bockstaele, E.: Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance, TrAC-Trend. Anal. Chem., 23, 535–552, https://doi.org/10.1016/j.trac.2004.04.001, 2004.
Thanigai Arul, K., Manikandan, E., Ladchumananandasivam, R., and Maaza, M.: Novel polyvinyl alcohol polymer based nanostructure with ferrites co-doped with nickel and cobalt ions for magneto-sensor application, Polym. Int., 65, 1482–1485, https://doi.org/10.1002/pi.5242, 2016.
Tsai, M.-C., Zhuang, D.-X., and Chen, P.-Y.: Electrodeposition of macroporous silver films from ionic liquids and assessment of these films in the electrocatalytic reduction of nitrate, Electrochim. Acta, 55, 1019–1027, https://doi.org/10.1016/j.electacta.2009.09.070, 2010.
Wakida, F. T. and Lerner, D. N.: Non-agricultural sources of groundwater nitrate: a review and case study, Water Res., 39, 3–16, https://doi.org/10.1016/j.watres.2004.07.026, 2005.
Wang, J., Zhang, Z., and Wang, S.: Facile fabrication of Ag/GO/Ti electrode by one-step electrodeposition for the enhanced cathodic reduction of nitrate pollution, Journal of Water Process Engineering, 40, 101839, https://doi.org/10.1016/j.jwpe.2020.101839, 2021a.
Wang, J., Li, Y., Pan, D., Han, H., and Zhang, P.: Self-assembly of silver nanoparticles on chitosan/polyvinylpyrrolidone modified micro-needle electrode for amperometric detection of nitrate in seawater, Microchem. J., 164, 105965, https://doi.org/10.1016/j.microc.2021.105965, 2021b.
Wang, L., Kim, J., and Cui, T.: Self-assembled graphene and copper nanoparticles composite sensor for nitrate determination, Microsyst. Technol., 24, 3623–3630, https://doi.org/10.1007/s00542-018-3792-7, 2018.
World Health Organization: Guidelines for drinking-water quality: incorporating 1st and 2nd addenda, Vol. 1, Recommendations, 3rd edn., World Health Organization, ISBN 9789241547611, 2006.
Zahran, M., Khalifa, Z., Zahran, M. A.-H., and Azzem, M. A.: Recent advances in silver nanoparticle-based electrochemical sensors for determining organic pollutants in water: A review, Materials Advances, 2, 7350–7365, https://doi.org/10.1039/D1MA00769F, 2021.
Zhad, H. R. L. Z. and Lai, R. Y.: Comparison of nanostructured silver-modified silver and carbon ultramicroelectrodes for electrochemical detection of nitrate, Anal. Chim. Acta, 892, 153–159, https://doi.org/10.1016/j.aca.2015.08.022, 2015.
Zhao, S., Tong, J., Li, Y., Sun, J., Bian, C., and Xia, S.: Palladium-gold modified ultramicro interdigital array electrode chip for nitrate detection in neutral water, Micromachines, 10, 223, https://doi.org/10.3390/mi10040223, 2019.
Zhou, D.-L., Zhang, Q.-L., Lv, Z.-Y., Chen, W.-Y., Liu, X.-F., Lu, Y.-H., Wang, A.-J., and Feng, J.-J.: Facile synthesis of a porous network-like silver film for electrocatalytic detection of nitrate, Microchim. Acta, 180, 1495–1500, https://doi.org/10.1007/s00604-013-1089-1, 2013.