the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Novel low-cost instrumentation based on an RGB sensor using molecularly imprinted polymers (MIPs) for the rapid detection of aqueous 2-methoxphenidine (2-MXP)
Serguei Stoukatch
Francois Dupont
Joseph W. Lowdon
Gil van Wissen
Kasper Eersels
Bart van Grinsven
Jean-Michel Redouté
In this paper, we demonstrate a novel, cost-effective sensing system utilizing a molecularly imprinted polymer (MIP) for the indirect colorimetric detection of 2-methoxphenidine (2-MXP). Unlike other colorimetric methods that often require expensive spectrometers and bulky read-out equipment, our system is streamlined, employing basic components such as a digital RGB colour sensor, a white LED, and a 3D-printed opaque enclosure compatible with standard spectrometer cuvettes. The sensor is constructed from readily available commercial components using conventional manufacturing processes. Our approach is versatile, accommodating various liquid analytes, making it suitable for diverse applications, including rapid toxicological screening. To this end, optimization towards the dwell time, number of assays needed, and a dose response for the methodology are explored. Specifically, we focus on the detection of 2-MXP in an aqueous solution within a concentration range of 0.05 to 1 mM. Within range, our system effectively identifies the presence of the analyte and quantifies its concentration. Notably, we achieved a detection limit as low as 0.026 mM, which corresponds to a typical metabolite concentration observed in humans. These results underscore the potential of our prototype sensor for practical applications in the rapid and economical field of diagnosis of MXP intoxication.
- Article
(2707 KB) - Full-text XML
- BibTeX
- EndNote
A recent report by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA, 2024) has highlighted the rise of new psychoactive substances (NPSs) in the EU (Varì et al., 2020). These designer drugs, structurally altered to bypass current legislation, are toxicologically similar to classic illicit drugs (Shafi et al., 2020). Diphenidine (WHO, 2020a) and its derivative methoxphenidine (MXP) (WHO, 2020b) are an example of such compounds and have emerged on the black market for recreational use, typically sold online. MXP, with ketamine-like dissociative effects at 50–100 mg doses, is often a key ingredient in NPS mixtures. Its pharmacological effects and toxicity are largely unknown, and its numerous regioisomers and derivatives (McLaughlin et al., 2016) complicate detection and identification, further hindered by chemical impurities. This is why the demand for detecting psychoactive substances like MXP in samples is high and growing, though analyses with traditional methods pose a challenge.
Common techniques (Woźniak et al., 2020) such as chromatography, infrared (IR), Raman, and mass spectroscopy often require complex sample preparation. To address these challenges, researchers are exploring alternative methods. Studies have shown that X-ray powder diffraction (XRPD) effectively detects psychoactive substances and outperforms IR and Raman spectroscopy in complex compounds (Jurásek et al., 2020, 2022). Successful identification of MXP isomers has also been achieved using gas and high-performance liquid chromatography with mass spectroscopy (McLaughlin et al., 2016). Additionally, new techniques like MALDI-Q-TOF-MS and UHPLC-MS are being developed for detecting MXP and its metabolites in biological samples (Vagnerova et al., 2022). Even though above-mentioned methods are highly effective and sensitive, they are expensive and require specialized laboratory equipment and highly skilled personal. Thus, there is an increasing demand for low-cost, rapid test kits that could be used in the field by, for instance, law enforcement agencies.
In this context, a valuable emerging alternative is the use of molecularly imprinted polymers (MIPs) (Ayerdurai et al., 2023; He et al., 2024). These polymers, due to their inherent affinity for the target analyte, can achieve selective targeting (Hua et al., 2023). For this reason (Martín-Esteban and Sellergren, 2012), MIPs have been utilized in a wide range of applications, in particular, in biosensors and chemosensors (Giancarla et al., 2023). MIP techniques effectively target small molecules (molecular weight < 1500 g mol−1) (Turner et al., 2006). They have been used for selective detection in various applications, such as melamine in milk (Caldara et al., 2022), antibiotics in milk (Bitas and Samanidou, 2018), and pesticides in environmental samples (Marć et al., 2021). MIP-based sensors offer advantages: a low price point, ease of implementation, robustness, and stability (Bahrani et al., 2021). Furthermore, MIPs are effective at biological concentration levels, making them suitable for detecting NPS in various scenarios (Musiał et al., 2022). Studies have demonstrated MIP-based detection of synthetic cathinones and amphetamines in urine (Sánchez-González et al., 2019) and 4-methyldimethcathinone (4-MDMC) (Chen et al., 2021), as well as MXP and its regioisomers (Lowdon et al., 2018). This said, one of the main limitations of MIP technology lies in the deposition of the receptors (Caldara et al., 2023), with a strong alternative being the utilization of MIP-based dye displacement assays that enable the indirect naked-eye detection of compounds. These assays involve competitive binding between the target molecule and a dye for binding sites within the MIP matrix. When the target molecule binds to the MIP, it displaces the dye, causing a measurable change in the absorbance of a solution, which correlates with the concentration of the target molecule in the sample. This approach enables sensitive and specific detection of analytes ranging from small molecules to biomacromolecules, depending on the design and synthesis of the MIPs. This methodology has already been highlighted for the detection of 2-MXP (Lowdon et al., 2019), amphetamine (Lowdon et al., 2021), and xylazine (Marroquin-Garcia et al., 2024). Researchers (Heidt et al., 2021; Goossens et al., 2023) have built upon these optical MIPs, providing microfluidic solutions coupled with spectrophotometers for the rapid on-site detection of compounds. This said, to date, colorimetric detection has tended to be performed using expensive commercial spectrophotometers (Ye et al., 2018; Wang et al., 2023), though it is possible to utilize low-cost components to achieve the same result.
This work therefore presents a novel, straightforward, and low-cost sensing system based on MIP particles deposited at the bottom of the cuvette for the indirect colorimetric detection of aqueous 2-MXP. This low-tech solution is characterized by a custom in-house low-cost photometer setup (Dupont et al., 2024) that employs an RGB sensor and an LED. Constructed from readily available commercial components, the system can be assembled in a common laboratory without the need for a cleanroom. Thus, the proposed methodology facilitates the cost-effective, sensitive (as low as 0.1 mM), and rapid colorimetric analysis of liquid analytes with simplistic equipment.
2.1 Reagents, components, and equipment
All chemicals and reagents were purchased from Sigma-Merck. All weights were measured on an analytical balance ACS 100-4M, from KERN (KERN, 2024), Germany. All solution volumes were measured using a one-channel Eppendorf manual mechanical pipette with 1000 µL retractable tips (epT.I.P.S.® Standard) (Eppendorf Research, 2024). All optical measurements were conducted inside a disposable plastic cuvette (Avantor by VWR, 2024) (VWR 612-5503) made of polymethyl methacrylate (PMMA) with dimensions of mm2. To manufacture all 3D-printed components, we employed the 3D printer Ultimaker S5 Pro (Ultimaker, 2024a) with recommendations by the vendor Ultimaker Tough PLA (polylactic acid), black, and 3D printing filament of 2.85 mm diameter (Ultimaker, 2024b). According to the literature (Avantor by VWR, 2024), the tensile Young's modulus of PLA is 3.25±1.19 GPa, the melting point is 151.8 °C, and the glass transition temperature (Tg) is 59.1 °C. PLA is a biocompatible and biodegradable plastic that has previously been used for similar applications (Diliën et al., 2017).
2.2 Synthesis and preparation of MIP-based assay
The methodology surrounding the synthesis of the MIPs for the detection of 2-MXP and their subsequent conversion into the dye displacement assay was taken directly from prior work (Lowdon et al., 2019). In short, methacrylic acid (MAA, 1.02 mmol), ethylene glycol dimethacrylate (EGDMA, 1.7 mmol), and azobisisobutyronitrile (AIBN, 0.30 mmol) were dissolved in dimethyl sulfoxide (DMSO) in the presence of 2-MXP (0.17 mmol). The mixture was purged with N2 and polymerized at 65 °C for 12 h. MIPs were milled using a Fritsch Planetary Micro Mill (Fritsch, 2024) and sieved to obtain microparticles smaller than 100 µm. A sieve was used for this process, with a 100 µm mesh, collecting any particles that passed through. The template molecule was removed by Soxhlet extraction (Pirzada and Altintas, 2021) with acetic acid and methanol (1:10 ), followed by methanol, and verified using FTIR. The resulting MIP powder was then dried at 60 °C overnight. Further, 20 mL of a 1 mM aqueous malachite green solution was added to 500 mg of MIP powder (microparticles smaller than 100 µm) and shaken at 250 rpm for 2 h. The suspension was then filtered, and the collected solid was washed with distilled water until the filtrate was colourless. The coloured MIP powder was then dried overnight at 60 °C, yielding the MIP-based dye displacement assay.
2.3 Colorimetric measurement system
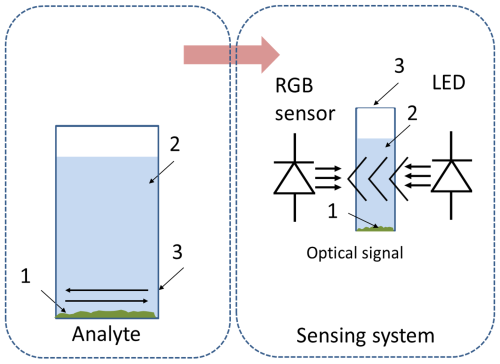
The colorimetric measurement system (Fig. 1) has been previously developed and comprehensively described in a prior study (Dupont et al., 2024). In essence, this system consists of a white LED (400–880 nm, 5500 K colour temperature, VLMW33S2V1-5K8L-08) and a digital RGB sensor (VEML3328, Vishay, Malvern, PA, USA), enclosed within a black 3D-printed opaque PLA housing designed to accommodate a commercially available plastic cuvette. These components were placed 15 mm above the bottom of the enclosure. To mitigate ambient light interference, the enclosure is equipped with an opaque cap. A complete list of components used in the colorimetric system is presented in Dupont et al. (2024).
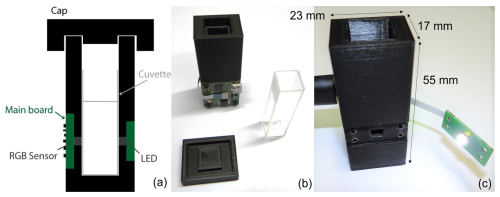
Figure 1Prototype overview: (a) cross-sectional schematic view; (b) frontside with main PCB, cuvette, and cap; and (c) backside with unmounted LED PCB. This figure was partially adapted from Dupont et al. (2024).
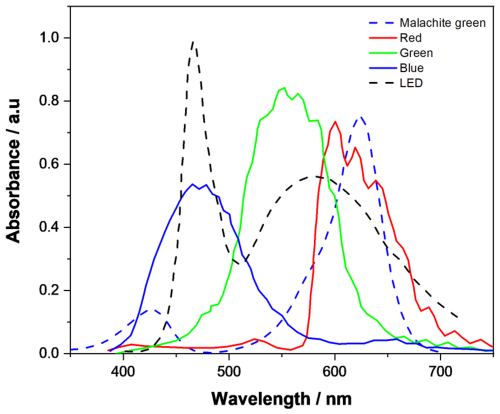
The sensor interface circuit was based on the SensorXplorer and VEML3328-SB development kits (Vishay, Malvern, PA, USA), with a customized layout tailored specifically for this application. Data collection was facilitated by the VEML3328_Xplorer_v1.4.1 software, which streamlined device configuration and data management. Figure 2 shows the RGB sensor channel normalized sensitivity alongside the absorbance spectrum of the malachite green dye. The red channel (with peak sensitivity at 610 nm) was the only channel that overlapped with the dye's maximum absorbance wavelength (λmax=621 nm; Mohseni et al., 2014). Therefore, the red channel was selected as the best choice for this analysis and was the only channel used in the remaining experiments. In principle, the system for this particular application can be further optimized; for example, the white LED can be replaced by a red LED, and the RGB sensor can be replaced by a corresponding photodiode with higher sensitivity to red light. However, in this study, we deliberately decided to maintain the generality of the system, suitable for detecting various dyes, in order to ensure the cost effectiveness of the developed system.
2.4 Analysis of aqueous samples
To assess the signal variation from the colorimetric measurement system, the MIP-based dye displacement assay was introduced into the plastic cuvette, followed by the addition of 2.5 mL of the analyte solution (2-MXP). The cuvette was then placed within the measurement system, where the RGB spectra were recorded (Fig. 3). As the assay is designed towards the colorimetric detection of 2-MXP, there should be trends observable when the assay is incubated with the displacement assay. This study investigated multiple parameters: the mass of the MIP assay applied (ranging from 2.5 to 15 mg), the time dependency of dwell time before conducting measurements (spanning 0 to 70 min), and the concentration of the analyte (0.05 to 0.5 mM).
3.1 Dwell time dependency study
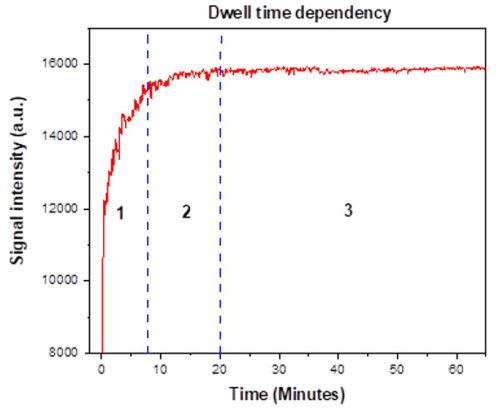
As the MIP utilized in the experiments is specifically engineered towards the detection of 2-MXP and preloaded with malachite green dye, exposure to aqueous 2-MXP results in the dye being released into the surrounding solution. Thus, this provides a robust mechanism of indirect colorimetric detection, only possible as the assay has been tailored towards 2-MXP. If the MIP were not specifically designed for 2-MXP detection, no dye displacement would occur in its presence. This has been highlighted in previous studies (Lowdon et al., 2018, 2019) where the cross-sensitivity of the detection method to other chemicals and contaminants in solutions is demonstrated to have a high degree of selectivity and sensitivity. Subsequently, the amount of malachite green displaced from the MIP directly correlates with the concentration of 2-MXP in the solution, with the dwell time and mass of assay directly affecting this. It can therefore be assumed that these factors could also impact on the sensing apparatus, with these being variables of high importance for consideration. Therefore, to ensure that the assay does not directly interfere and diffract the light being passed through the cuvette, impacting the measurements, the dwell time of sample analysis was investigated. To this end, the RGB signal was monitored as a function of time after applying a known volume of analyte inside the cuvette that contained the MIP-based assay. The experiment was then conducted over 70 min, monitoring the stability of the resultant signal (Fig. 4). Figure 4 clearly illustrates that the RGB signal output changes significantly within the first 8 min (section 1 of the plot). During this time, most of the large MIP particles, which have the greatest impact on the light transmission between the LED and the RGB sensor, gradually settle to the bottom of the cuvette. Between 8 and 20 min (section 2), the number of residual particles in suspension, likely the smallest ones, decreases significantly, causing the signal to nearly stabilize. After 20 min (section 3), the RGB signal stabilizes, and visual confirmation showed no remaining MIP particles in suspension. For rapid analyses, the RGB signal can be reliably used after the first 8 min (section 2), provided that the system remains stable, and no shaking or vibration occurs. However, for the most accurate data, a dwell time of 20 min (section 3) is recommended. It is important to note that, as outlined in Dupont et al. (2024), the particles deposited at the bottom of the cuvette do not affect the RGB sensor output.
3.2 Mass dependency study
To study the impact that the mass of assay has on the sensitivity of the platform, an experiment was devised where 2.5 mL aliquots of varying concentrations (0.05–1 mM) were added to different masses (2.5–12.5 mg) of MIP powder within the cuvette. From these experiments the mass of MIP-based assay could be plotted against the concentration of 2-MXP, generating a matrix that could be referred to for the perceived detection of the analyte (Fig. 5).
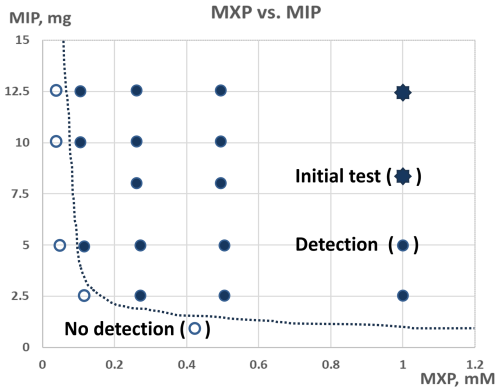
Figure 5A plot demonstrating the mass of MIP-based assay against the concentration of 2-MXP in solution and the subsequent perceived detectability.
The plot features two distinct zones, separated by a dashed line: the “detection” zone and the “no detection” zone. The detection zone indicates that the target concentration of 2-MXP can be identified with the appropriate amount of MIP, while the no detection zone signifies that 2-MXP cannot be detected regardless of the MIP quantity used. The initial test result is marked with an eight-point star. Points within the detection zone are represented by filled dots, whereas those outside are shown with unfilled dots. This plot is useful for estimating the amount of MIP needed to detect various 2-MXP concentrations. As anticipated, higher concentrations of 2-MXP require less MIP, while lower concentrations necessitate more. For instance, detecting a 1 mM 2-MXP concentration requires only 2.5 mg of MIP, whereas detecting a 0.1 mM concentration requires at least 12.5 mg of MIP. Additionally, using a smaller amount of MIP can be advantageous because fewer particles settle more quickly, leading to a more stable RGB signal.
3.3 Concentration-dependent studies
As demonstrated in Sect 3, a dwell time of 20 min provides the most accurate results. Therefore, the studies were conducted with a 20 min duration as follows. Two reference cuvettes were prepared: one with 2.5 mL of water and another with 2.5 mL of water containing 12.5 mg of MIP powder. The purpose of the latter was to verify that particles settled at the bottom of the cuvette do not significantly impact the sensor readings. Additionally, four cuvettes were prepared with varying concentrations of the analyte 2-MXP: 0.5, 0.25, 0.1, and 0.05 mM, as shown in Fig. 6.
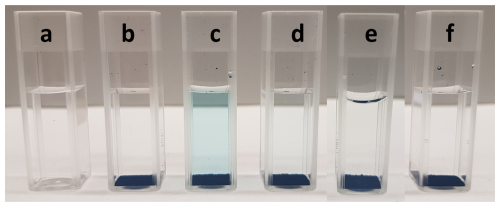
Figure 6A reference cuvette with 2.5 mL of water (a), another reference cuvette with 2.5 mL of water and 12.5 mg of MIP powder (b), and four cuvettes with 12.5 mg of MIP powder and varying 2-MXP concentrations: 0.5 mM (c), 0.25 mM (d), 0.1 mM (e), and 0.05 mM (f).
Visual inspection revealed that cuvettes a and b showed no dye displacement, while cuvettes c through f exhibited decreasing levels of displaced malachite green dye as the analyte concentration decreased. This trend was further confirmed with the RGB setup, which clearly differentiated between the various 2-MXP concentrations (Fig. 7). Among the concentrations tested, 0.05 mM showed a signal intensity comparable to water, with a distinguishable signal first appearing at 0.1 mM. At 0.25 mM, the amount of displaced dye increased noticeably, and at 0.5 mM, there was a marked exponential increase in dye displacement.
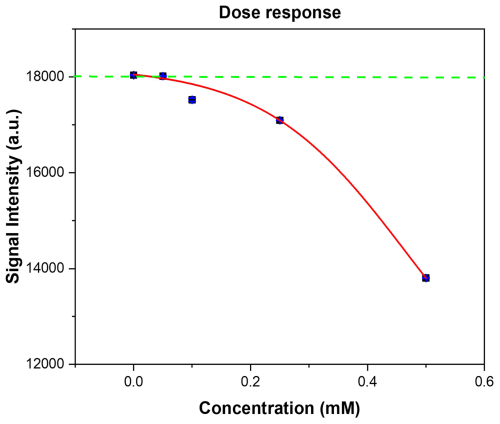
Figure 7The RGB signal output stimulated from the increasing absorbance of the displacement of malachite green from 12.5 mg of assay against the exposure concentration of 2-MXP (0.05–0.5 mM). Each point represents the average of 50 RGB sensor readings. and the standard deviation is less than 2 % of the average (blue lines).
The resulting dose response graph was plotted using Origin x and the data analysed with a dose response ()) fitting. The resulting trend (red line) line yielded an R2 of 0.99962, demonstrating a strong correlation between the amount of dye displaced and the signal intensity observed. Due to the extremely low internal standard deviation of the results, the theoretical limit of detection (LoD) of the sensor is 0.021 mM when calculated using the 3σ methodology. This is well within the calculated physiological range that typically occurs during human metabolism (Elliott et al., 2015) after ingesting a standard dose of 2-MXP (from 75 to 120 mg) (WHO, 2020a) and therefore highlights the sensitivity of the system.
In this work, a simple and cost-effective MIP-based sensing system for the indirect colorimetric detection of 2-methoxphenidine (2-MXP) was demonstrated. Unlike typical colorimetric systems that rely on spectrometers, this system utilizes an RGB sensor and a corresponding LED. Constructed from readily available commercial components, the system can be manufactured in a standard laboratory environment using conventional industrial methods, without the need for a cleanroom.
The developed colorimetric measurement system, based on RGB sensors and MIP receptors, effectively detects 2-MXP in aqueous solutions at concentrations below 1 mM. It can distinguish between concentrations of 1, 0.5, 0.25, and 0.1 mM 2-MXP. The analysis time is 20 min, primarily due to the time required for the MIP powder to settle at the bottom of the cuvette. This sediment does not affect the measurement system's output signal, as confirmed by previous studies.
Additionally, the study established the detection window for MIP–MXP interactions, specifying the amount of MIP needed to detect various 2-MXP concentrations. The tested concentration range (from 1 to 0.1 mM) is relevant for real-life applications, as it corresponds to concentrations found in human metabolism after ingesting typical oral doses of 2-MXP. This MIP-based sensing method is thus suitable for detecting a broad range of specific liquid analytes associated with human metabolism.
Data sets are available upon request by contacting the correspondence author.
Conceptualization: SeS, FD, JWL, GVW, KE, BVG, and JMR; methodology: SeS, FD, JWL, and GVW; software: FD; validation: SeS, FD, JWL, and GVW; formal analysis: SeS, FD, JWL, and GVW; investigation: SeS, FD, JWL, and GVW; resources: KE, BVG, and JMR; data curation: SeS, FD, and JWL; writing and preparation of the draft: SeS, FD, and JWL; review and editing: SeS, FD, JWL, GVW, KE, BVG, and JMR; visualization: SeS, FD, JWL, and GVW; supervision: FD, JWL, KE, BVG, and JMR; project administration: FD and KE.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The work has been carried out in the framework of the Interreg EMR Food Screening project (Food Screening – EMR 159). It was supported by Interreg Euregio Meuse-Rhin, the Walloon Region of Belgium, and the Ministerie van Economische Zaken en Klimaat. The whole project was also supported by Provincie Limburg and Die Landesregierung Nordrhein-Westfalen. This project was carried out in collaboration with a consortium of research institutions and small- and medium-sized enterprises (SMEs) in Belgium, the Netherlands, and Germany.
This research has been supported by Interreg Euregio Meuse-Rhin, the Walloon Region of Belgium, and the Ministerie van Economische Zaken en Klimaat in the framework of the Interreg Food Screening – EMR 159 project. The whole project was also supported by Provincie Limburg and Die Landesregierung Nordrhein-Westfalen. This open-access publication was funded by the University of Liège.
This paper was edited by Bernhard Jakoby and reviewed by two anonymous referees.
Avantor by VWR: Disposable macro-cuvettes, https://si.vwr.com/store/product/18616117/disposable-macro-cuvettes (last access: 19 September 2024), 2024.
Ayerdurai, V., Cieplak, M., and Kutner, W.: Molecularly imprinted polymer-based electrochemical sensors for food contaminants determination, Trends Anal. Chem., 158, 116830, https://doi.org/10.1016/j.trac.2022.116830, 2023.
Bahrani, S., Aslani, R., Hashemi, S. A., Mousavi, S. M., and Ghaedi, M.: Chapter 7 – Introduction to molecularly imprinted polymer, in: Interface Science and Technology, Vol. 33, edited by; Ghaedi, M., Elsevier, 511–556, https://doi.org/10.1016/B978-0-12-818805-7.00006-0, 2021.
Bitas, D. and Samanidou, V.: Molecularly imprinted polymers as extracting media for the chromatographic determination of antibiotics in milk, Molecules, 23, 316, https://doi.org/10.3390/molecules23020316, 2018.
Caldara, M., Lowdon, J. W., Royakkers, J., Peeters, M., Cleij, T. J., Diliën, H., Eersels, K., and van Grinsven, B.: A Molecularly Imprinted Polymer-Based Thermal Sensor for the Selective Detection of Melamine in Milk Samples, Foods, 11, 2906, https://doi.org/10.3390/foods11182906, 2022.
Caldara, M., van Wissen, G., Cleij, T. J., Diliën, H., van Grinsven, B., Eersels, K., and Lowdon, J. W.: Deposition Methods for the Integration of Molecularly Imprinted Polymers (MIPs) in Sensor Applications, Adv. Sen. Res., 202200059, 2751–1219, https://doi.org/10.1002/adsr.202200059, 2023.
Chen, H., Wu, F., Xu, Y., Liu, Y., Song, L., Chen, X., He, Q., Liu, W., Han, Q., Zhang, Z., Zou, Y., and Liu, W.: Synthesis, characterization, and evaluation of selective molecularly imprinted polymers for the fast determination of synthetic cathinones, RSC Adv., 11, 29752–29761, https://doi.org/10.1039/d1ra01330k, 2021.
Diliën, H., Peeters, M., Royakkers, J., Harings, J., Cornelis, P., Wagner, P., Steen Redeker, E., Banks, C. E., Eersels, K., van Grinsven, B., and Cleij, T. J.: Label-free detection of small organic molecules by molecularly imprinted polymer functionalized thermocouples: toward in vivo applications, ACS Sensors, 2, 583–589, https://doi.org/10.1021/acssensors.7b00104, 2017.
Dupont, F., Stoukatch, S., Laurent, P., Eersels, K., van Grinsven, B., and Redouté, J.-M.: Design Aspects for Portable LED-Based Colorimetric Characterisation Systems Targeting Liquid Analytes, MDPI Sensors, 24, 1960, https://doi.org/10.3390/s24061960, 2024.
Elliott, S. P., Brandt, S. D., Wallach, J., Morris, H., and Kavanagh, P. V.: First reported fatalities associated with the `research chemical' 2-methoxydiphenidine, J. Anal. Toxicol., 39, 287–293, https://doi.org/10.1093/jat/bkv006, 2015.
EMCDDA – European Monitoring Centre for Drugs and Drug Addiction: Document Library, https://www.emcdda.europa.eu/drugs-library/jwh-018-oxizids-structural-evolution-synthetic-cannabinoids (last access: 19 September 2024), 2024.
Eppendorf Research: Mechanical Pipette, https://www.eppendorf.com/be-en/eShop-Products/Liquid-Handling/Manual-Pipetting-Dispensing/Eppendorf-Research-plus-p-PF-534798 (last access: 19 September 2024), 2024.
Fritsch: Planetary Micro Mill, https://www.fritsch-international.com/sample-preparation/milling/planetary-mills/ (last access: 19 September 2024), 2024.
Giancarla, A., Zanoni,C., Spina, S., Magnaghi, L. R., and Biesuz, R.: Trends in Molecularly Imprinted Polymers (MIPs)-Based Plasmonic Sensors, Chemosensors, 11, 144, https://doi.org/10.3390/chemosensors11020144, 2023.
Goossens, J., Vandenryt, T., Lowdon, J. W., van Grinsven, B., Eersels, K., Deferme, W., and Thoelenet, R.: Design of a Lab-on-Chip Cartridge for the Optical Detection of Small Molecules Based on Dye-Displacement MIPs, IEEE T. Instrument. Meas., 72, 1–9, https://doi.org/10.1109/TIM.2023.3301040, 2023.
He, X., Ji, W., Xing, S., Feng, Z., Li, H., Lu, S., Du, K., and Li. X.: Emerging trends in sensors based on molecular imprinting technology: Harnessing smartphones for portable detection and recognition, Talanta, 268, 125283, https://doi.org/10.1016/j.talanta.2023.125283, 2024.
Heidt, B., Rogosic, R., Leoné, N., Brás, E. J. S., Cleij, T. J., Harings, J. A. W., Diliën, H., Eersels, K., and van Grinsven, B.: Topographical Vacuum Sealing of 3D-Printed Multiplanar Microfluidic Structures, Biosensors, 15, 395, https://doi.org/10.3390/bios11100395, 2021.
Hua, Y., Kukkar, D., Brown, R. J. C., and Kim, K.-H.: Recent advances in the synthesis of and sensing applications for metal-organic framework-molecularly imprinted polymer (MOF-MIP) composites, Crit. Rev. Environ. Sci. Technol., 53, 258–289, https://doi.org/10.1080/10643389.2022.2050161, 2023.
Jurásek, B., Bartůněk, V., Huber, Š., Fagan, P., Setnička, V., Králík, F., Dehaen, W., Svozil, D., and Kuchař, M.: Can X-Ray Powder Diffraction Be a Suitable Forensic Method for Illicit Drug Identification?, Front. Chem., 2020, 499, https://doi.org/10.3389/fchem.2020.00499, 2020.
Jurásek, B., Rimpelová, S., Babor, M., Čejka, J., Bartůněk, V., and Kuchař, M.: Intriguing Cytotoxicity of the Street Dissociative Anesthetic Methoxphenidine: Unexpected Impurities Spotted, Int. J. Mol. Sci., 23, 2083, https://doi.org/10.3390/ijms23042083, 2022.
KERN: Analytical balances, https://www.kern-sohn.com/shop/en/laboratory-balances/analytical-balances/ABS-N_ABJ-NM_ACS_ACJ/ (last access: 19 September 2024), 2024.
Lowdon J. W., Alkirkit, S. M. O., Mewis, R. E., Fulton, D., Banks, C. E., Sutcliffe, O. B., and Peeters, M.: Engineering molecularly imprinted polymers (MIPs) for the selective extraction and quantification of the Novel Psychoactive Substance (NPS) methoxphenidine and its regioisomers, Analyst, 143, 2002–2007, https://doi.org/10.1039/C8AN00131F, 2018.
Lowdon, J. W., Eersels, K., Rogosic, R.; Boonen, T., Heidt, B., Diliën, H., van Grinsven, B., and Cleij, T. J.: Surface grafted molecularly imprinted polymeric receptor layers for thermal detection of the New Psychoactive substance 2-methoxphenidine, Sens. Actuat. A, 295, 586–595, https://doi.org/10.1016/j.sna.2019.06.029, 2019.
Lowdon, J. W., Diliën, H., van Grinsven, B., Eersels, K., and Cleij, T. J.: Colorimetric Sensing of Amoxicillin Facilitated by Molecularly Imprinted Polymers, Polymers, 13, 2221, https://doi.org/10.3390/polym13132221, 2021.
Marć, M., Bystrzanowska, M., Pokajewicz, K., and Tobiszewski, M.: Multivariate assessment of procedures for molecularly imprinted polymer synthesis for pesticides detehttps://doi.org/10.3390/ma14227078, 2021.
Marroquin-Garcia, R., van Wissen, G., Cleij, T. J., Eersels, K., van Grinsven, B., and Diliën, H.: Single-use dye displacement colorimetry assay based on molecularly imprinted polymers: Towards fast and on-site detection of xylazine in alcoholic beverages, Food Control, 161, 110403, https://doi.org/10.1016/j.foodcont.2024.110403, 2024.
Martín-Esteban, A. and Sellergren, B.: 2.17 – Molecularly Imprinted Polymers, in: Comprehensive Sampling and Sample Preparation, edited by: Pawliszyn, J., Academic Press, 331–344, https://doi.org/10.1016/B978-0-12-381373-2.00046-6, 2012.
McLaughlin, G., Morris, N., Kavanagh, P. V., Power, J. D., O'Brien, J., Talbot, B., Elliott, S. P., Wallach, J., Hoang, K., Morris, H., and Brandt, S. D.: Test purchase, synthesis, and characterization of 2-methoxydiphenidine (MXP) and differentiation from its meta- and para-substituted isomers, Drug Test Anal., 8, 98–109, https://doi.org/10.1002/dta.1800, 2016.
Mohseni, N., Bahram, M., and Olivieri, A. C.: Second-order advantage obtained from standard addition first-order instrumental data and multivariate curve resolution-alternating least squares. Calculation of the feasible bands of results, Spectrochim. Acta A, 122, 721–30, https://doi.org/10.1016/j.saa.2013.11.073, 2014.
Musiał, J., Czarny, J., and Gadzała-Kopciuch, R.: Overview of analytical methods for determining novel psychoactive substances, drugs and their metabolites in biological samples, Crit. Rev. Toxicol., 52, 239–258, https://doi.org/10.1080/10408444.2022.2091424, 2022.
Pirzada, M. and Altintas, Z.: Chapter 14 – Template Removal in Molecular Imprinting: Principles, Strategies, and Challenges, in: Molecular Imprinting for Nanosensors and Other Sensing Applications, edited by: Denizli, A., Elsevier, 367–406, https://doi.org/10.1016/B978-0-12-822117-4.00014-9, 2021.
Sánchez-González, J., Odoardi, S., Bermejo, A. M., Bermejo-Barrera, P., Romolo, F. S., Moreda-Piñeiro, A., and Strano-Rossi, S.: HPLC-MS/MS combined with membrane-protected molecularly imprinted polymer micro-solid-phase extraction for synthetic cathinones monitoring in urine, Drug Test. Anal. 11, 33–44, https://doi.org/10.1002/dta.2448, 2019.
Shafi, A, Berry, A. J., and Sumnall, H.: Wood DM, Tracy DK. New psychoactive substances: a review and updates, Ther. Adv. Psychopharmacol., 17, 2045125320967197, https://doi.org/10.1177/2045125320967197, 2020.
Turner, N. W., Jeans, C. W., Brain, K .R., Allender, C. J., Hlady, V., and Britt, D. W.: From 3D to 2D: a review of the molecular imprinting of proteins, Biotechnol. Prog., 22, 1474–1489, https://doi.org/10.1021/bp060122g, 2006.
Ultimaker: 3D printers, UltiMaker S5, https://ultimaker.com/3d-printers/s-series/ultimaker-s5/ (last access: 19 September 2024), 2024a.
Ultimaker: Tough PLA material properties, https://ultimaker.com/materials/s-series-tough-pla/ (last access: 19 September 2024), 2024b.
Vagnerova, M., Kolderova, N., Jurasek, B., Sykora, D., and Kuchar, M.: Novel method for determination of methoxphenidine and its main metabolite in biological samples, Toxicologie Analytique et Clinique, 34, S175, https://doi.org/10.1016/j.toxac.2022.06.302, 2022.
Varì, M. R., Mannocchi, G., Tittarelli, R., Campanozzi, L. L., Nittari, G., Feola, A., Umani Ronchi, F., and Ricci, G.: New Psychoactive Substances: Evolution in the Exchange of Information and Innovative Legal Responses in the European Union, Int. J. Environ. Res. Publ. Health, 17, 8704, https://doi.org/10.3390/ijerph17228704, 2020.
Wang, S., Xu, S., Zhou, Q., Liu, Z., and Xu, Z.: State-of-the-art molecular imprinted colorimetric sensors and their on-site inspecting applications, J. Sep. Sci., 46, e2201059, https://doi.org/10.1002/jssc.202201059, 2023.
WHO – World Health Organization: Critical Review Report: Diphenidine, https://cdn.who.int/media/docs/default-source/controlled-substances/43rd-ecdd/diphenidine-report-complete.pdf?sfvrsn=4f7ad16d_2 (last access: 19 September 2024), 2020a.
WHO – World Health Organization: Critical Review Report: 2-MEO-diphenidine, 2-MXP, https://www.who.int/docs/default-source/controlled-substances/43rd-ecdd/2-meo-diphenidine-report-final-a.pdf (last access: 19 September 2024), 2020b.
Woźniak, M. K., Banaszkiewicz, L., Wiergowski, M., Tomczak, E., Kata, M., Szpiech, B., Namieśnik, J., and Biziuk, M.: Development and validation of a GC–MS/MS method for the determination of 11 amphetamines and 34 synthetic cathinones in whole blood, Forensic Toxicol., 38, 42–58, https://doi.org/10.1007/s11419-019-00485-y, 2020.
Ye, T., Yin, W., Zhu, N., Yuan, M., Cao, H., Yu, J., Gou, Z., Wang, X., Zhu, H., Reyihanguli, A., and Xu, F.: Colorimetric detection of pyrethroid metabolite by using surface molecularly imprinted polymer, Sensors Actuat. B, 254, 417–423, https://doi.org/10.1016/j.talanta.2021.122397, 2018.