the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Surface plasmon assisted toxic chemical NO2 gas sensor by Au ∕ ZnO functional thin films
Ravinder Gaur
Himanshu Mohan Padhy
Manikandan Elayaperumal
In this short communication, we propose a surface plasmon resonance (SPR) sensor based on a ZnO Au hybrid thin-film material structure and experimentally investigate its sensitivity improvement. The Kretschmann-based SPR sensor utilizes ZnO thin films and nanostructures for performance enhancement. The advancement in SPR technology relies on a low-cost, high-sensitivity, and high-selectivity sensor. Metal oxide (MO) has been incorporated into the SPR sensor to be used for detection of biological and chemical compounds. ZnO as one of the metal oxides is an attractive material due to its unique physical and optical properties. Numerous techniques for fabrication and characterization of ZnO on SPR gold substrate have been studied. The mechanism for gas and biomolecule detection depends on their interaction with the ZnO surface, which is mainly attributed to the high isoelectric point of ZnO. There are several types of ZnO nanostructures which have been employed for SPR application based on the Kretschmann configuration. In the future, the thin film and nanostructures of ZnO could be a potential application for miniature design, robust, high sensitivity, and a low-cost portable type of SPR biosensor to be used for on-site testing in a real-time and label-free manner. The present work includes the application of a developed SPR setup for gas sensing at room temperature using a specially designed gas cell. The change in the optical properties of dielectric layers (ZnO) with adsorption of gases (NO2) in order to develop an optical sensor has been presented. The obtained results emphasize the applications of an SPR setup for the study of interaction of adsorbed gas molecules, with dielectrics and gas sensing.
- Article
(3872 KB) - Full-text XML
- BibTeX
- EndNote
Surface plasmon resonance (SPR) is the resonance for free electrons with collective oscillations in the metal surface when excited by the incident light, and the energy of the incident light would be absorbed by free electrons during the resonance, so that the energy of the output light would be decreased to some extent (Sharma et al., 2012; Homola et al., 1999; Nikitin et al., 1999). The decreasing degree of the output light is related to the incident light, and the SPR angle is the incident light as the output light is decreased to the minimum. The SPR can be excited in a variety of ways, such as prism coupling, grating coupling, and optical fiber coupling, and SPR has been extensively used in sensing of various physical, chemical, and biochemical parameters. The surface plasmon resonance biosensor (SPR biosensor) has a remarkable capability of real-time detection and monitoring of biomolecules (Nikitin et al., 1999; Wilde et al., 1997; Skalska et al., 2010; Dostálek et al., 2006; Kumar et al., 2014). There are various factors that significantly affect the function of the SPR biosensor, which are needed to be considered for designing a stable and highly sensitive SPR biosensor. The two features, the high detection sensitivity and the good SPR peak for better precision, are critical to the function implementation of the SPR biosensor as well as its application. The SPR biosensor has been studied through the past 30 years.
Conductometric gas sensors exploiting semiconductor sensing materials are exclusively used for fabricating gas sensors due to a simple detection principle and easy sensor fabrication (Sharma et al., 2012; Homola et al., 1999; Nikitin et al., 1999; Wilde et al., 1997; Skalska et al., 2010; Dostálek et al., 2006; Kumar et al., 2014; Manikandan et al., 2016; Thanigai Arul et al., 2016; Paulraj et al., 2020; Saasa et al., 2015; Gaur et al., 2021; Paulraj et al., 2021). However, these types of sensors come with a few disadvantages, such as the requirement of a high operating temperature which results in consumption of high power with a poor selectivity. This paves the way for realizing efficient gas sensors which can operate at room temperature which is provided by optical sensors (Homola et al., 1999). SPR-based sensors have several merits, like simple fabrication, room temperature operation, and fast response at lower concentrations of toxic gases. Many researchers have exploited SPR-based sensors in Kretschmann configuration for detecting toxic gases by coating a suitable sensing film on the plasmonic metal (Nikitin et al., 1999; Wilde et al., 1997). The deposition of a gas-sensitive layer on the noble metal surface (excites surface plasmons (SPs) at the metal–dielectric interface) is the major requirement for SPR-based gas sensors whose refractive index changes in contact with the target gas, in turn bringing the variations in resonance parameters which are in correlation with the interacting gas molecule concentration.
NO2 is a highly toxic gas dangerously affecting human health and also contributing to the formation of photochemical smog. The major sources of NO2 gas include automobiles, thunderstorms, gas boilers, and industries (Skalska et al., 2010). Increase in the levels of NO2 gas even after 20 ppm concentration is injurious to living beings as it can affect the upper respiratory tract and lungs badly (Dostálek et al., 2006). Thus, it is an urgent requirement to fabricate a highly efficient and sensitive NO2 gas sensor.
The SPR-based NO2 gas sensor requires the identification of a suitable sensitive coating. Wide band gap semiconductor materials such as SnO2, WO3, and ZnO are the suitable sensing materials which have been utilized extensively for realization of conductometric gas sensors for the detection of a number of toxic, dangerous and harmful gases, including NO2, SO2, CO, H2S, and CO2 for domestic, commercial, and industrial applications (Kumar et al., 2014; Mane et al., 2014; Cao et al., 2013). Amongst all the metal oxides, ZnO is an exclusive material with semiconducting, photo-conducting, piezoelectric, and pyroelectric properties suitable for applications in gas sensors (Hossain et al., 2005). Therefore, in the present work ZnO has been chosen as a sensing material for detection of NO2 gas utilizing the SPR technique.
In the present work, variation in optical properties of gas-sensitive thin film (ZnO) with interaction of toxic gas (NO2) have been monitored. The suitable sensing film, i.e., ZnO thin film, is deposited on Au-coated BK7 glass prisms using the radio frequency (R.F.) magnetron sputtering technique. SPR reflectance curves are obtained for different concentrations of NO2 gas for the prism Au ZnO system in angular interrogation mode. The prepared sensor structure is investigated for selectivity corresponding to other interfering and interacting gases.
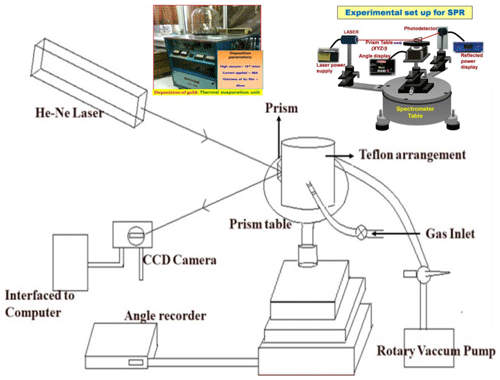
An angular interrogation method has been exploited to record the SPR reflectance in Kretschmann configuration where Au thin film (around 40 nm) was deposited on a BK-7 glass prism (right angled) to support the propagation of SPs at the prism Au interface. An indigenously assembled SPR measurement system with a p-polarized light source (He–Ne laser light of wavelength 633 nm) was used to excite the SPs for the prism Au ZnO air system. The reflected light from another face of the prism was monitored using a photodetector interface with the power meter as a function of the incident angle. The schematic of the laboratory-assembled SPR measurement system specially designed for gas-sensing applications is shown in Fig. 1, where a gas cell specially designed is placed on the prism table. The gas cell is provided with a window to couple the Au prism and gas-sensitive (ZnO) films so that the ZnO thin film is in the vicinity of the target gas molecules inside the gas cell. The complete scan of the SPR reflectance curve was acquired by inserting a particular concentration of the target gas of gas-sensing measurements. For the transient measurements, the photodetector is replaced by CCD where the whole prism Au ZnO system is fixed at a stage where the incident angle corresponds to the resonance angle and continuous variation in reflected intensity was recorded with increase in gas concentration. In order for the sensor (prism Au ZnO) to recover, a rotary pump is exploited to evacuate the chamber and fill in the fresh clean air. The selectivity of the sensor is also a crucial parameter which is observed by monitoring the interference with other gases, including H2, NH3, and LPG.
Table 1Physical parameter for the growth of the ZnO thin film. Growth using R.F. magnetron sputtering.
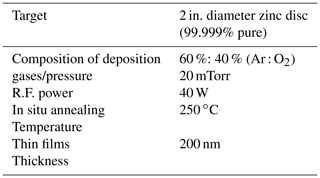
All the technical information regarding the growth of the ZnO thin film is given in Table 1, where the films were prepared by using R.F. magnetron sputtering with a constant thickness of 200 nm on an Au-coated glass prism for sensing applications and the experimental schematic figure shown in Fig. 1.
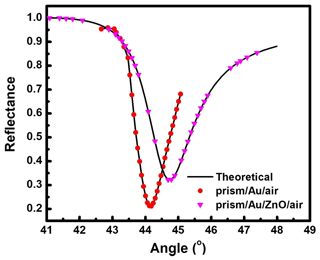
Figure 2SPR reflectance curve for prism Au air and prism Au ZnO air systems at 633 nm excitation wavelength.
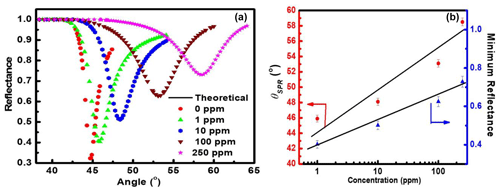
Figure 3(a) Variation of reflectance with incident angle (i.e., SPR reflectance curve) for the prism Au ZnO structure on interaction with NO2 gas of fixed concentrations varying from 1 to 250 ppm and (b) linear change in SPR angle (θSPR) and minimum reflectance with varying concentration of NO2 gas (calibration curve).
The primary application of ZnO thin film for the SPR sensor was as a sensing layer for gas detection. Generally, selection of material for sensing a particular gas is determined by the interaction of its surface-active side formed by ions O−, O2−, H+, and OH− with gas molecules. An SPR-based gas sensor using ZnO is widely used for sensing for both reductive and oxidative gases. The key property of ZnO for gas sensing is the reversible interaction of its surface with the gas molecule due to covalent bonding or dipole–dipole interaction. As an n-type semiconductor, ZnO has a high level of defects to provide a good surface for gas molecule absorption. As the molecules of gas are absorbed on the ZnO surface, changes in the dielectric constant of the ZnO take place and result in refractive index changes. Figure 2 shows the experimental SPR reflectance curves (symbols) obtained for prism Au air and prism Au ZnO air systems at a wavelength λ= 633 nm. The narrow SPR reflectance curve represents low absorption losses along with the longer propagation length of SPs, which is quantified by real and imaginary parts of the refractive index, i.e., n and k, respectively (Raether, 1988). Gold possesses stable optical and chemical properties, making it the most preferred metal for SP excitation at the metal–dielectric interface (Hutter et al., 2001). The prism Au air system as evident from Fig. 2 shows the resonance angle at 44.14∘ at Rmin (reflectance at the resonance position) of 0.213. The continuous curve shown in Fig. 2 indicates the theoretically fitted curve obtained by Fresnel's equations (Kooyman et al., 2008). The dielectric constant of air and glass prisms and the thickness of metal thin films have been used in the fitting. The values of dielectric parameters () and refractive indexes (n+ik) of metal thin films are estimated by fitting the experimental SPR data (symbols in Fig. 2). The estimated values fairly correspond to the reported values (Bendavid et al., 1999) shown in Fig. 2. The reflectance data were recorded by the SPR setup in the angular interrogation mode using Kretschmann configuration. The SPR curve shows a shift in θSPR at higher angles after integrating the thin film of ZnO with a prism Au structure. The observed shift in θSPR is due to a change in the interface from Au air to Au dielectric with another layer of ZnO of different dielectric constants.
The observed SPR reflectance curve (symbols) for prism Au ZnO system at λ= 633 nm is the experimental SPR data fitted with Fresnel's equations while using the estimated value of the dielectric constant for 40 nm thin Au film () and varying the value of the dielectric constant of the respective ZnO thin film as fitting parameters. For ZnO thin films, the values of the real part of the complex dielectric constant (ε′) and refractive index (n) were estimated at an incident higher wavelength of λ= 633 nm to be 3.24 and 1.8, respectively (Saha et al., 2009).
3.1 Response to NO2 gas obtained by the prism Au ZnO as-prepared structure
The as-prepared sensor structure (i.e., prism Au ZnO air system) with 200 nm thin ZnO film grown at 20 mTorr sputtering pressure is used for measuring the response to NO2 gas utilizing the SPR technique. Figure 3a shows the SPR reflectance data obtained for prism Au WO3 structure at λ= 633 nm at room temperature on exposure to the increasing concentration from 1 to 250 ppm of NO2 gas. The SPR reflectance curve for the prism Au ZnO structure obtained in the ambient air (0 ppm) is also included in Fig. 3a for comparison. Linear variation in SPR angle (θSPR) and minimum reflectance (Rmin) with various concentrations of NO2 gas are plotted in Fig. 3b as a function of NO2 gas concentration, and it was observed that the values of both θSPR and Rmin were found to increase linearly from 44.8 to 58.5∘ and from 0.32 to 0.73, respectively (Fig. 3b).
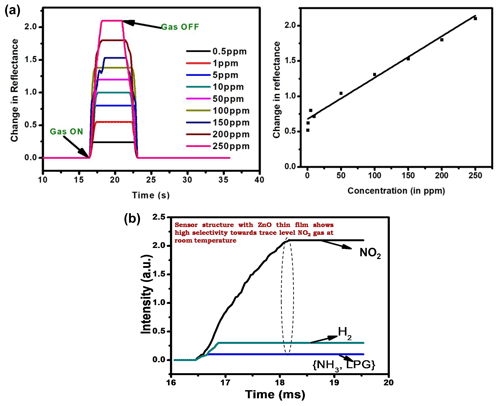
Figure 5Panel (b) shows the linear variation in the sensing response (ΔR) with the concentration of target gas (i.e., calibration curve of the sensor prism Au ZnO). The selectivity response of the prepared sensor is shown in Fig. 6, where the gas-sensing response is measured for other harmful gases like NH3, LPG, and H2 of 250 ppm concentration.
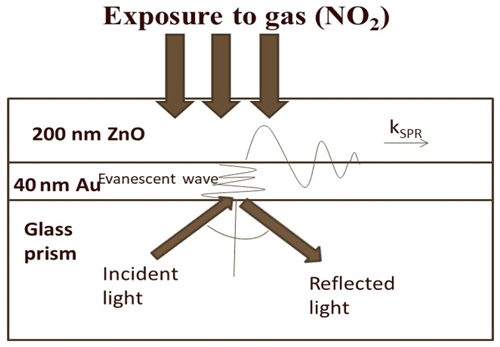
This is because of the change in the optical properties of the sensing dielectric layer (i.e., ZnO thin film) in terms of refractive index due to adsorption of oxidizing NO2 gas. The sensitivity of the NO2 gas sensor using the ZnO gold sensing layer was estimated to be 0.05±0.0004∘/ppm from Fig. 3b. The basic mechanism behind the detection of toxic chemical gases by the SPR-based optical gas sensor is explained clearly in Fig. 4. It is evident from the figure that SPR sensors work on the principle of change in SP dispersion conditions, which may be due to differences in the refractive index of two things: bulk media (i.e., change of air to NO2 gas) and dielectric layer, i.e., ZnO thin film and related hybrid metal oxides explored recently (Maciak et al., 2005; Manikandan et al., 2016; Thanigai Arul et al., 2016; Paulraj et al., 2020; Saasa et al., 2015).
3.2 Transient response of the prepared sensor structure: prism Au ZnO
The gas-sensing measurements made in the dynamic mode shown in Fig. 5a indicate the change in reflectance for the prism Au ZnO system in the resonance position for varying concentrations from 0.5 to 250 ppm. The response cycle as evident from the figure is shown by the rise in the reflectance value (at fixed θ) to a stable high value (saturation) after the insertion of target gas molecules. From Fig. 5a, the sensor regains its original value of minimum reflectance (Rmin) just after removing the target gas (i.e., NO2 gas) from the gas cell during the recovery cycle.
Recently, recent work has been attempted for the same research group, with various metal oxides, dielectric matrix groups, polymerics blended with metals, and SPR-based metals core-shell structures used to detect the different chemical gas vapors (Manikandan et al., 2016; Thanigai Arul et al., 2016; Paulraj et al., 2020; Saasa et al., 2015; Gaur et al., 2021; Paulraj et al., 2021).
The SPR technique has been effectively used for monitoring the change in dielectric properties of semiconducting hybrid thin films (ZnO Au) on exposure to NO2 gas for fabricating an efficient gas sensor. A high sensing response is obtained using ZnO thin film of thickness of about 200 nm with increase in the concentration range from 0.5 to 250 ppm of NO2 gas. The prepared optical gas sensor indicates the quick response (1 s) and also the recovery towards the target gas. The sensitivity of the prepared sensor is found to be 0.091∘/ppm, and selectivity studies were done with other gases like NH3, LPG, and H2. The measurement of gas sensing in real time also confirmed that our SPR device responded the NO2 gas. Even this system needs to be improved to obtain more systematic results of the selectivity, sensitivity, reversibility, and stability; these results open up the possibility of the NO2 gas detection using a ZnO Au-based SPR sensor at room temperature.
This research has used Origin Scientific Software, available at https://www.originlab.com/ (OriginLab, 2020) for mathematical graphical purposes.
The data will be made available upon request to the corresponding author.
RG initiated the experiment to form the thin films by the sputtering unit. HMP assisted in the materials collection and ideas for a further stage of the experiment. EM compiled the results analysis and data processing to make a full shape of the manuscript write-up.
The authors declare that they have no conflict of interest.
The authors are thankful to the University of Delhi for providing a facility to carry out the research work.
This paper was edited by Nobutito Imanaka and reviewed by two anonymous referees.
Bendavid, A., Martina, P. J., and Wieczorek, L.: Morphology and optical properties of gold thin films prepared by filtered arc deposition, Thin Solid Films, 354, 169–175, https://doi.org/10.1016/S0040-6090(99)00434-4, 1999.
Cao, Y., Jia, D., Wang, R., and Luo, J.: Rapid one-step room-temperature solid-state synthesis and formation mechanism of ZnO nanorods as H2 S-sensing materials, Solid State Electron., 82, 67–71, https://doi.org/10.1016/j.sse.2012.12.015, 2013.
Dostálek, J., Ladd, J., Jiang, S., and Homola, J.: SPR biosensors for detection of biological and chemical analytes, in: Springer Series on Chemical Sensors and Biosensors, edited by: Wolfbeis, O. S., Springer, Berlin, 177–190, https://doi.org/10.1007/5346_019, 2006.
Gaur, R., Manikandan, P., Manikandan, D., Umapathy, S., Padhy, H. M., Maaza, M., and Manikandan, E.: Noble Metal Ion Embedded Nanocomposite Glass Materials for Optical Functionality of UV–Visible Surface Plasmon Resonance (SPR) Surface-Enhanced Raman Scattering (SERS) X-ray and Electron Microscopic Studies: An Overview, Plasmoncs, 12, 1–33, https://doi.org/10.1007/s11468-021-01413-w, 2021.
Homola, J., Yee, S. S., and Gauglitz, G.: Surface plasmon resonance sensors: Review, Sensor Actuat. B, 54, 3–15, https://doi.org/10.1016/S0925-4005(98)00321-9, 1999.
Hossain, M. K., Ghosh, S. C., Boontongkong, Y., Thanachayanont, C., and Dutta, J.: Growth of Zinc Oxide Nanowires and Nanobelts for Gas Sensing Applications, J. Metastab. Nanocryst., 23, 27–30, https://doi.org/10.4028/www.scientific.net/JMNM.23.27, 2005.
Hutter, E., Fendler, J. H., and Roy, D.: Surface Plasmon Resonance Studies of Gold and Silver Nanoparticles Linked to Gold and Silver Substrates by 2-Aminoethanethiol and 1,6-Hexanedithiol, J. Phys. Chem. B, 105, 11159–11168, https://doi.org/10.1039/C5CP06121K, 2001.
Kooyman, R. P. H.: Physics of Surface Plasmon Resonance, in: Surface Plasmon Resonance, edited by: Schasfoort, R. B. M. and Tudos, A. J., RSC Publishing, Cambridge, 15–34, https://doi.org/10.1039/9781788010283-00027, 2008.
Kumar, M., Kumar, A., and Abhyankar, A. C.: SnO2 based sensors with improved sensitivity and response-recovery time, Ceram. Int., 40, 84118418, https://doi.org/10.1016/j.ceramint.2014.01.050, 2014.
Maciak, E., Opilski, Z., and Pustelny, T.: Effect of humidity on NH2 gas sensitivity of Nafion WO3 sensing structure of SPR sensor, Mol. Quantum Acoust., 26, 205–215, 2005.
Mane, A. T., Kulkarni, S. B., Navale, S. T., Ghanwat, A. A., Shinde, N. M., Kim, H.-H., and Patil, V. B.: NO2 sensing properties of nanostructured tungsten oxide thin films, Ceram. Int., 40, 16495–16502, https://doi.org/10.1016/j.ceramint.2014.08.001, 2014.
Manikandan, E., Lokesh, K., Kavitha, G., Mani, G., John Bosco, B. R., Rasiah, L., and Maaza, M.: Effective ammonia detection using n-ZnO/p-NiO heterostructured nanofibers, IEEE Sens. J., 16, 2477–2483, https://doi.org/10.1109/JSEN.2016.2517085, 2016.
Nikitin, P. I., Beloglazov, A. A., Kochergin, V. E., Valeiko, M. V., and Ksenevich, T. I.: Surface plasmon resonance interferometry for biological and chemical sensing, Sensor Actuat. B,, 54, 43–50, https://doi.org/10.1016/S0925-4005(98)00325-6, 1999.
OriginLab: https://www.originlab.com/, last access: 18 November 2020.
Paulraj, P., Umar, A., Rajendran, K., A., Kumar, R., Manikandan, E., Pandian, K., and Baskoutas, S.: Solid-state synthesis of Ag-doped PANI nanocomposites for their end-use as an electrochemical sensor for hydrogen peroxide and dopamine, Electrochim. Ac., 363, 137158, https://doi.org/10.1016/j.electacta.2020.137158, 2020.
Paulraj, P., Umar, A., Rajendran, K., Manikandan, A., Sathamraja, A., Kumar, R., Manikandan, E., Pandian, K., Baskoutas, S., Algadi, H., Ibrahim, A. A., and Alsaiari, M. A.: Methylene blue intercalated layered MnO2 nanosheets for high-sensitive non-enzymatic ascorbic acid sensor, J. Mater. Sci.-Mater. El., 32, 8317–8329, https://doi.org/10.1007/s10854-021-05391-x, 2021.
Raether, H.: Surface Plasmons on smooth and rough surfaces and on Gratings, Verlag Berlin Hiedelberg, Springer Tokyo, https://doi.org/10.1007/BFb0048319, 1988.
Saasa, V., Mokwena, M., Dhonge, B. P., Manikandan, E., Kennedy, J., Murmu, P., Dewar, J., Erasmus, R., Whaley, M., Mukwevho, E., and Mwakikunga, B.: Optical and Structural Properties of Multi-wall-carbon- nanotube-modified ZnO Synthesized at Varying Substrate Temperatures for Highly Efficient Light Sensing Devices, Sensors & Transducers, 195, 9–17, 2015.
Saha, S., Mehan, N., Sreenivas, K., and Gupta, V.: Temperature dependent optical properties of (002) oriented ZnO thin film using surface plasmon resonance, Appl. Phys. Lett., 95, 071106, https://doi.org/10.1063/1.3206954, 2009.
Sharma, A., Tomar, M., and Gupta, V.: Low temperature operating SnO2 thin film sensor loaded with WO3 micro-discs with enhanced response for NO2 gas, Sensor Actuat. B, 161, 1114–1118, https://doi.org/10.1016/j.snb.2011.10.014, 2012.
Skalska, K., Miller, J. S., and Ledakowicz, S.: Trends in NOx abatement: A review, Sci. Total Environ., 408, 3976–3989, https://doi.org/10.1016/j.scitotenv.2010.06.001, 2010.
Thanigai Arul, K., Manikandan, E., Ladchumananandasivam, R., and Maaza, M.: Novel polyvinyl alcohol polymer-based nanostructure with ferrites co-doped with nickel and cobalt ions for magneto-sensor application, Polym. Int., 65, 1482–1485, https://doi.org/10.1002/pi.5242, 2016.
Wilde, J. N., Petty, M. C., Saffell, J., Tempore, A., and Valli, L.: Surface Plasmon Resonance Imaging for Gas Sensing, Meas. Control, 30, 269–272, https://doi.org/10.1177/002029409703000903, 1997.