the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Thin-film SnO2 and ZnO detectors of hydrogen peroxide vapors
Vladimir Aroutiounian
Valeri Arakelyan
Mikayel Aleksanyan
Gohar Shahnazaryan
Petr Kacer
Pavel Picha
Jiri Kovarik
Jakub Pekarek
Berndt Joost
Thin-film hydrogen peroxide vapor sensors made from Co-doped SnO2 and La-doped ZnO were manufactured using the high-frequency magnetron sputtering method. Thicknesses of deposited doped metal oxide films were measured and their morphology was investigated. The gas sensing characteristics of the prepared sensors were measured at different concentrations of hydrogen peroxide vapors and different operating temperatures of the sensor. It was found that both sensors made from doped metal oxides SnO2 and ZnO exhibit a sufficient response to 10 ppm of hydrogen peroxide vapors at the 200 and 220 ∘C operating temperature, respectively. It was established that the dependencies of the response on hydrogen peroxide vapor concentration have a linear character for prepared structures at the 150 ∘C operating temperature and can be used for determination of hydrogen peroxide vapor concentration.
- Article
(1709 KB) - Full-text XML
- BibTeX
- EndNote
Hydrogen peroxide (H2O2) is a chemical compound which is widely used in such fields as medicine, pharmacology, food and textiles due to the wide spectrum of its antibacterial properties, low toxicity and ecological purity (products of H2O2 decomposition are neutral to water and oxygen). However, pure H2O2 at large concentrations is explosive under certain conditions (for example, in the presence of transition metals). Therefore, concentrated solutions of H2O2 can cause burns in the case of contact with the skin, mucous membranes and respiratory tract. H2O2 is subsumed under the category of matter that is dangerous for man after a certain maximum permissible concentration. Therefore, the development of sensors for detection of H2O2 and determination of its concentration in the environment is important and is attracting the interest of chemists, physicians, engineers, etc. The H2O2 stable sensors can be used in analytical chemistry, in various fields of industry (food, wood pulp textile, pharmacology), in environmental control, in clinical diagnostics for prompt and reliable specification of diagnoses of different diseases and in checking of a course of treatment.
Several techniques such as chemiluminescence (Hsu et al., 2015), spectrophotometry (Rahim et al., 2011), fluorimetric and colorimetric detection (Sun et al., 2016), liquid chromatography, and electroanalytical and optical interferometry (Ensafi et al., 2016) have been developed for a reliable and sensitive determination of H2O2. These techniques are complex, expensive and time-consuming. Now electrochemical sensors are used (Chen et al., 2013, 2014; Lin and Chang, 2015). A large range of materials such as ferric hexacyanoferrate (Prussian blue) and other metal hexacyanoferrates, metallophthalocyanines and metalloporphyrins, transition metals and metal oxides are used for the manufacturing of these sensors (Puganova and Karyakin, 2005; Chen et al., 2012). The advantages of these sensors are simplicity of manufacturing, good response and capability of control in real time. In recent years, the progress of nanotechnology has promoted advances in the field of manufacturing of the H2O2 electrochemical sensors. For example, carbon nanotubes and graphene can be used either as substrates with high specific areas for catalytic materials or as electrocatalysts by themselves (Šljukič et al., 2006; Pogacean et al., 2015; Wu et al., 2017; Yang et al., 2017).
Hydrogen peroxide serves as a disinfectant for medical equipment and surfaces as well as for sterilizing of surgical instruments. Therefore, the correct selection of the H2O2 concentration during the sterilization of the equipment technological surfaces and also control of the H2O2 content in air after completion of the disinfection cycle are very important. Note that the process of chemical decontamination can be carried out in two different ways: the first one is a wet approach using water or any other solutions of H2O2 (certain concentration) and the second one is a dry method using H2O2 in the vapor phase (Kačer et al., 2012). Therefore, the development and manufacturing of stable and reproducible sensors sensitive to hydrogen peroxide vapors (HPV) are urgently required (Taizo et al., 1998; Oberländer et al., 2014). The checking of the HPV phase is also crucially significant in connection with counterterrorism efforts. The sensors sensitive to HPV may find application in the detection of peroxide-based explosives (Bohrer et al., 2008; Mills et al., 2009).
The most used method is based on the determination of the concentration of HPV after cooling down and being absorbed in the water. The near-infrared spectrophotometry was used for the monitoring of the concentration of HPV in the course of sterilization (Corveleyn et al., 1997). The chemiresistive films made from organic p-type semiconducting phthalocyanines metalized with elements of p-, d-, and f-blocks were sensitive to HPV (Bohrer et al., 2008). An amperometric sensor for detection of HPV made of an agarose-coated Prussian-blue modified thick-film screen-printed carbon-electrode transducer was investigated (Benedet et al., 2009). An organic single-wire optical sensor for HPV made of organic core/sheath nanowires with a wave guiding core and chemiluminogenic cladding was reported on (Zheng et al., 2012).
The aim of the present paper is the development of technology, manufacturing and investigation of solid-state HPV sensors made from semiconductor-doped metal oxide nanostructured films. The paper is divided into four sections. In Sect. 1 the necessities in the development of the H2O2 sensors and, in particular, sensors sensitive to HPV, are briefly described. The material characteristics and the manufacturing technology of sensors made from semiconductor Co-doped SnO2 and La-doped ZnO nanostructures are reported in Sect. 2. The results of the investigations of response for prepared HPV sensors are presented in Sect. 3. The conclusions are drawn and the directions of future work are described in the final section.
Ceramic targets made from Co-doped SnO2 and La-doped ZnO metal oxides were synthesized by the method of solid-phase reaction in the air. The powders of initial oxides (SnO2, ZnO and dopant Co2O3 and La2O3) were weighed in the applicable quantities to obtain targets with specified compositions: SnO2 doped with 2 at. % Co, ZnO doped with 1 at. % La and ZnO doped with 2 at. % La. These mixtures were carefully intermixed and pressed. The compacted samples SnO2 < Co >were exposed to thermal treatment into the programmable furnace Nabertherm, HT O4/16 with the controller C 42. The annealing of the compacted samples SnO2 < Co >was carried out at 500, 700, 1000 and 1100 ∘C, consecutively, soaking at each temperature during 5 h. The following program of annealing for the compacted samples of ZnO < La >was chosen: rising of the temperature from room temperature up to 1300 ∘C for 3 h, soaking at this temperature during 4 h, and further decrease in the temperature for 3 h prior to room temperature. Then, the synthesized compositions were subjected to mechanical treatment in the air in order to eliminate surface defects. Thus, smooth, parallel targets with a diameter ∼ 40 mm and thickness ∼ 2 mm were manufactured.
Chemical composition of prepared targets was studied using a NitonTM XL 3t GOLDD+ XRF Analyzer. The results of these investigations have shown that the real content of cobalt's atoms was equal to 1.23 at. % for the ceramic target with a specified composition of SnO2+ 2 at. % Co. The real content of lanthanum atoms was equal to 0.71 and 1.47 at. % for the targets with a specified composition of ZnO + 1 at. % La and ZnO + 2 at. % La, respectively. So ceramic targets with compositions of Sn0.9877Co0.0123O2, Zn0.9929La0.0071O, and Zn0.9853La0.0147O were synthesized.
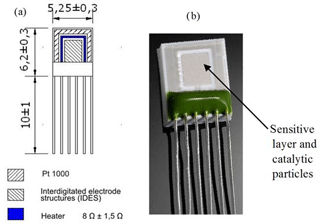
Figure 1The structure of the Multi-Sensor-Platform (a) and the sensor based on the Multi-Sensor-Platform (b).
The prepared SnO2 < Co >and ZnO < La >targets had sufficient conductance and were used for deposition of thin films using the high-frequency magnetron sputtering method. Alumina and the Multi-Sensor-Platform (purchased from TESLA BLATNÁ, Czech Republic) were used as substrates for nanosized films. The following working conditions of the high-frequency magnetron sputtering were chosen: the power of the magnetron generator unit was equal to 60 W; the substrate temperature during sputtering was equal to 200 ∘C; the distance between substrate and target was equal to 7 cm. The duration of the sputtering process was equal to 20 min for Co-doped SnO2 films. The sputtering process of La-doped ZnO compositions was carried out during 15, 20 and 30 min for preparing films with different thicknesses.
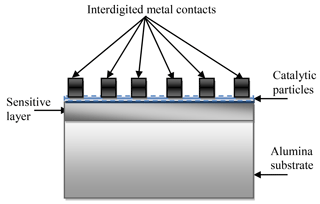
In the case when the Multi-Sensor-Platforms were used as substrates, the chip can be kept at constant temperature using heat resistance. The structure of the Multi-Sensor-Platform is shown in Fig. 1a. The platform integrates a temperature sensor (Pt 1000), a heater and interdigitated electrode structures with a platinum thin film on a ceramic substrate. The heater and the temperature sensor are covered with an insulating glass layer. A gas-sensitive layer made of SnO2 < Co >or ZnO < La >compositions was deposited onto the non-passivated electrode structures using the high-frequency magnetron sputtering method. The Multi-Sensor-Platforms are converted into gas sensors that way (Fig. 1b). The sensing device was completed through the ion-beam sputtering deposition of palladium catalytic particles (the deposition time ∼ 3 s). Further heat treatment of the manufactured structures in the air was carried out at 250 ∘C temperature to obtain homogeneous films and eliminate mechanical stresses.
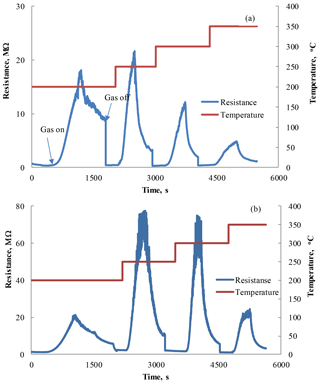
Figure 4Response–recovery curves observed under the influence of 1800 ppm HPV (42–45 % RH) measured at different operating temperatures for the Zn0.9929La0.0071O sensors with film thicknesses of 80 (a) and 210 nm (b).
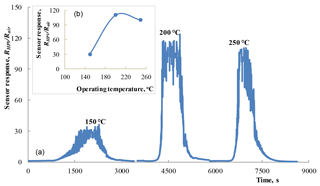
Figure 5(a) Response–recovery curves observed under the influence of 100 ppm HPV (42–45 % RH) measured at different operating temperatures for the SnO2 < Co >sensor. Dependence of the sensor response on operating temperature (b).
The structure of the sensor on the alumina substrate is schematically shown in Fig. 2. In this case the interdigitated metal contacts were deposited by the ion-beam sputtering method after deposition of the gas-sensitive layer and catalytic particles. Interdigitated gold contacts (the deposition time was 1 h) and titanium contacts (the deposition time was 50 min) were used for Co-doped SnO2 and La-doped ZnO sensing layers, respectively. In this case the process of preparing the sensors on the alumina substrate is also finalized by an additional annealing of the structures in the air at the temperature of 250 ∘C during 2 h.
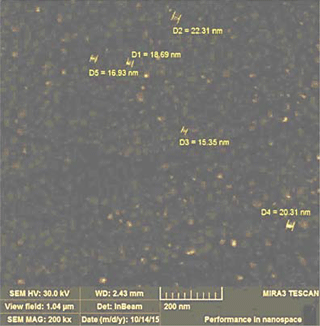
The morphology of the deposited doped metal oxide films was studied and the grain sizes of the films were determined using scanning electron microscopy (SEM) Mira 3 LMH (Tescan). For example, the SEM image of La-doped ZnO film is presented in Fig. 3. The average size of nanoparticles was equal to 18.7 nm for deposited films.
The thicknesses of the deposited doped metal oxide films were measured by an Ambios XP-1 profilometer. The thickness of the SnO2 < Co >film deposited on the Multi-Sensor-Platform was equal to 160 nm. The films made of the ZnO < La >had thicknesses in the range of 30–210 nm depending on the duration of the sputtering process.
The sensors with Co-doped SnO2 and La-doped ZnO-sensitive films manufactured by us are a resistive type. The operation of this type of sensor is based on the changes in the electrical resistance of a gas-sensitive semiconductor layer under the influence of HPV due to an exchange of charges between the molecules of adsorbed HPV and the semiconductor film.
The H2O2 decomposes to produce water vapors and oxygen:
These adsorbed oxygen molecules capture the electrons from the semiconductor film:
The change in the electrical resistance of the sensor takes place as a result of such exchange of electrons. This change in the sensor resistance under the influence of a target gas was recorded as a sensor sensing characteristic.
The gas sensing properties of the prepared resistive type gas sensors made from doped metal oxide films under the influence of HPV were investigated at Yerevan State University using an internally developed and computer-controlled static gas sensor test system (see Aroutiounian et al., 2013; Adamyan et al., 2018). The sensor was placed in a hermetic chamber. A certain quantity of H2O2 water solution (10 mg) was injected into the measurement chamber. Different concentrations of HPV (from 100 up to 4000 ppm) were reached in the chamber depending on the percentage content of the H2O2 water solution.
The measurements of the manufactured sensors' electrical resistance under the HPV influence were carried out at different operating temperatures (from room temperature up to 350 ∘C). The platinum heater located around the active surface of the sensor on the Multi-Sensor-Platform ensures a necessary temperature of the working body. The sensor on the alumina substrate is placed on the heater, which allows the temperature of the sensor's working body to rise up to 350 ∘C. All measurements of the electrical resistance were carried out at 0.5 V DC voltage applied on the sensor's electrode.
The typical response–recovery curves obtained as a result of these measurements for two sensors with a Zn0.9929La0.0071O sensitive layer are presented in Fig. 4. These films were deposited on an alumina substrate during 15 (Fig. 4a) and 30 min (Fig. 4b) and their thicknesses were equal to 80 and 210 nm, respectively. These characteristics demonstrate the change in the sensor's electrical resistance under the influence of 1800 ppm HPV at different operating temperatures.
As a result of the measurements of sensing characteristics, the sensor response was calculated as the ratio RHPV∕Rair, where RHPV is the sensor electrical resistance in the HPV atmosphere and Rair is the sensor resistance in the air without HPV. The results of such calculations of response for the SnO2 < Co >sensor are presented in Fig. 5. These measurements were carried out under the influence of 100 ppm HPV at different working body temperatures.
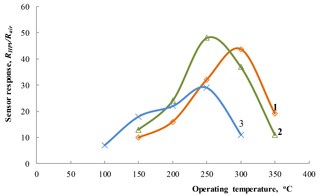
Figure 6Dependence of the response to 1800 ppm of HPV on operating temperature for the Zn0.9929La0.0071O sensors on an alumina substrate with film thicknesses of 80 (1) and 210 nm (2) and for the sensor with Zn0.9853La0.0147O films deposited on the Multi-Sensor-Platform (3).
The results of investigations of the dependence of the sensor response on operating temperature for sensors with the La-doped ZnO gas-sensitive layer are presented in Fig. 6. The concentration of target gas was 1800 ppm in these measurements. At a relatively low operating temperature (150 ∘C), the best response was observed for the structure with larger contents of impurity (Zn0.9853La0.0147O). At higher temperatures, a sensor with more thicker film shows a larger response. Probably a longer sputtering time allows a thicker film with a more perfect structure to be obtained. Besides, the roughness of the films' surfaces is the same since these sensitive layers were made under identical conditions. However, the working volume and, accordingly, the number of H2O2 molecules participating in the charge exchange process are larger for a thicker film.
Note that the electrical resistance of the prepared ZnO < La >sensors has changed in order of magnitude under the influence of HPV starting at an operating temperature of 100 ∘C. However, a longer time is needed for recovery of the sensor parameters at such a temperature. The pulsed rise in the working body temperature is needed for decreasing the recovery time of the investigated sensors. The response and recovery times were determined when the time required for reaching the 90 % resistance changes from the corresponding steady-state value of each signal. For a SnO2 < Co >structure both the response and recovery times were equal to 5 min at the temperatures more than 200 ∘C. For the ZnO < La >sensors the response and recovery times were on average equal to 6–8 and 10–12 min, respectively, at the operating temperatures more than 200 ∘C. The real response times may be less than the mentioned values. This is due to the fact that, as was already noted, 10 mg of an aqueous solution with a certain percentage content of H2O2 are injected into a measuring chamber in order to obtain the appropriate concentration of HPV. The response time of the sensor, calculated from the moment when the H2O2 water solution is injected into the chamber until the maximum response reaches 90 %, also includes the time necessary for the complete evaporation of the aqueous solution.
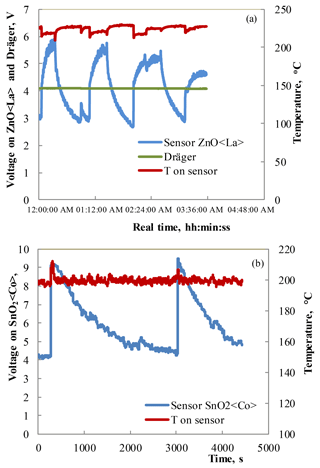
Figure 7(a) Response–recovery curves observed under the influence of 10 ppm HPV measured at 220 ∘C operating temperatures for the Zn0.9929La0.0071O sensor and Dräger sensor. (b) Response–recovery curves observed under the influence of 75 ppm HPV measured at 200 ∘C operating temperatures for the SnO2 < Co >sensor.
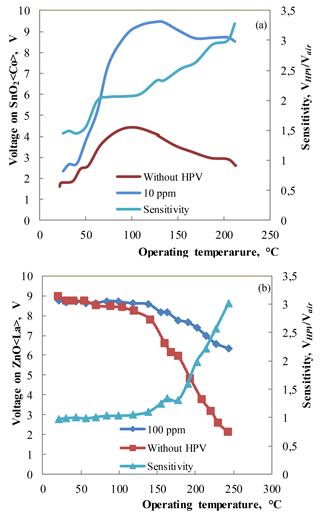
Figure 8(a) The temperature dependencies of voltage on sensor and sensitivity (VHPV∕Vair) for the SnO2 < Co >sensor measured under the influence of 10 ppm HPV (20–23 % RH) at 200 ∘C operating temperature. (b) The temperature dependencies of voltage on sensor and sensitivity (VHPV∕Vair) for the Zn0.9929La0.0071O sensor measured under the influence of 100 ppm HPV at 220 ∘C operating temperature.
As shown in Figs. 5 and 6, the sensor response decreases for both structures, when the temperature of a working body exceeds a certain value (250–300 and 200 ∘C for La-doped ZnO and Co-doped SnO2 sensors, respectively). The number of vapor molecules adsorbed on a surface and generally kept by Van der Waals forces (physical adsorption) decreases with the increasing temperature. More intensive exchange of electrons between the absorber and the absorbed molecules takes place when the stronger chemical nature bond is established between them, originated at capping of electronic shells of both adsorbent and adsorbate atoms. The number of chemisorbed centers increases with increasing temperature. Desorption prevails over the adsorption when a temperature is increased above a certain value and, therefore, the sensor response decreases. The temperature of the sensors, made of a ZnO < La >structure, above which the sensitivity decreases, is greater than for the sensors made of a SnO2 < Co >structure. Probably, the chemical bonds between molecules of ZnO and H2O2 are stronger than that between molecules of SnO2 and H2O2. The fact that the recovery time for sensors made of Co-doped SnO2 is less than that for La-doped ZnO sensors also testifies to what is mentioned above.
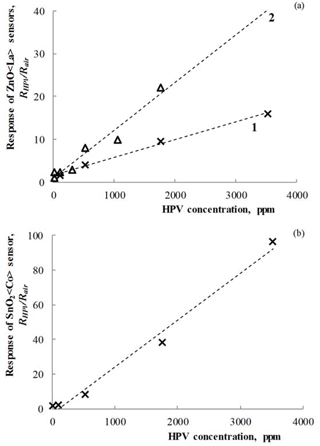
Figure 9Dependencies of the response on HPV concentration measured at 150 ∘C operating temperature for (a) Zn0.9929La0.0071O (1), Zn0.9853La0.0147O (2) and Sn0.9877Co0.0123O2 (b) sensors.
As has already been noted, the H2O2 belongs to the type of materials dangerous for man after a certain maximum permissible concentration. The permissible limit of exposure of 1,0 ppm has been established by the Occupational Safety and Health Administration (OSHA, USA; Corveleyn et al., 1997; Oberländer et al., 2014). It is immediately dangerous for life and health when its concentration reaches 75 ppm (Sun et al., 2016). Therefore, it was necessary to investigate the gas sensing characteristics of prepared sensors made from doped metal oxide films at low concentrations of HPV. Such measurements of the sensing properties of the prepared sensors with La-doped ZnO and Co-doped SnO2 sensitive films deposited on Multi-Sensor-Platforms were carried out at concentrations of HPV of less than 100 ppm at the University of Chemistry and Technology (Prague, CZ). The results of these investigations are presented in Figs. 7 and 8.
The measurements of the sensing characteristics of the sensors with a Zn0.9929La0.0071O sensitive layer to 10 ppm HPV were carried out in the following way. Firstly, an atmosphere containing 10 ppm of HPV was prepared in a laboratory model of an isolator. This HPV concentration decreased by spontaneous decomposition of H2O2. When a reference device (Dräger Sensor® H2O2 HC) could not detect any HPV, the ZnO < La >sensor was inserted into the model isolator. Then, the sensor responded immediately. When the maximum response was reached, the sensor was taken out into an atmosphere without any traces of HPV. This process was repeated three times (Fig. 7a). In these studies, a voltage on sensor at direct current is used as a parameter for sensing characteristics. The measurements of the sensing characteristics under the influence of 75 ppm HPV were carried out using the same method for the SnO2 < Co >sensors (Fig. 7b).
The temperature dependence of sensing parameter (or voltage on sensor) under the influence of 10 ppm HPV was investigated for the SnO2 < Co >sensors. For these measurements the atmosphere in the “Peroxybox” system developed in the same institute in Prague was controlled (0–10 ppm HPV and 20–23 % RH) and the sensor's temperature was changed. The final sensitivity was calculated as the voltage on sensor in “Peroxybox” system VHPV divided by voltage on sensor in the air Vair (Fig. 8a). The temperature dependence of sensing parameter under the influence of 100 ppm HPV was investigated using the same way for the ZnO < La >sensors (Fig. 8b).
The investigations of the prepared sensors under the influence of low concentrations of HPV showed that the sensitivity (VHPV/Vair) to 10 ppm of HPV was equal to ∼ 2 for the ZnO < La>sensors at the working body temperature of 220 ∘C. Note that the DrägerSensor® H2O2 HC reference device was not sensitive to 10 ppm of HPV (Fig. 7a). The investigations of the sensor sensitivity to very low concentrations (0–10 ppm) of HPV showed that the structure made of SnO2 < Co >exhibits a response to 10 ppm of HPV at the operating temperature starting from 50 ∘C (Fig. 8a). The sensitivity to 10 ppm of HPV was equal to ∼ 3 for the SnO2 < Co >sensors at the working body temperature of 200 ∘C.
Figure 9 presents the results of the investigations of the response at the different concentrations of HPV for the prepared sensors.
As can be seen in Fig. 9, the dependencies of sensor response on HPV concentration have a linear character for all sensors. Due to the linear dependence of the response on concentration of target gas, it is possible to determine HPV concentration in the environment.
The technology for the manufacturing of semiconductor sensors made from Co-doped SnO2 and La-doped ZnO nanostructured films was developed. The gas-sensitive Zn0.9929La0.0071O, Zn0.9853La0.0147O and Sn0.9877Co0.0123O2 layers were deposited onto alumina substrates and the Multi-Sensor-Platforms using the high-frequency magnetron sputtering method. Thicknesses of deposited doped metal oxide films were measured and their morphology was investigated. The thickness of the deposited films was in the range of 30–210 nm; the average size of nanoparticles was equal to 18.7 nm. Specimens detecting HPV were manufactured and investigated. The response of the prepared sensors was measured at different temperatures of the sensor working body and different concentrations of HPV. It was found that both Co-doped SnO2 and La-doped ZnO sensors exhibit a good response to HPV starting at 100 ∘C operating temperature. Sensors made from SnO2 < Co >and ZnO < La >were sufficiently sensitive to 10 ppm of HPV. It was established that the dependencies of the response on HPV concentration at the operating temperature of 150 ∘C have a linear character for prepared structures and can be used for determination of HPV concentration. Our future work will be directed to the long-time stabilization of sensor parameters and the improvement of such characteristics as operation speed and recovery time.
No data sets were used in this article.
The authors declare that they have no conflict of interest.
This article is part of the special issue “Sensor/IRS2 2017”. It is a result of the AMA Conferences, Nuremberg, Germany, 30 May–1 June 2017.
This investigation was supported by the Swiss National Science Foundation
(FNSNF) within the framework of the SCOPES DecoComp project. The authors
express gratitude to Vazgen Kuzanyan
for help in the measurements of thickness of our samples.
Edited by: Peter A. Lieberzeit
Reviewed by:
three anonymous referees
Adamyan, Z., Sayunts, A., Aroutiounian, V., Khachaturyan, E., Vrnata, M., Fitl, P., and Vlcek, J.: Nanocomposite sensors of propylene glycol, dimethylformamide and formaldehyde vapors, J. Sens. Sens. Syst., 7, 31–41, https://doi.org/10.5194/jsss-7-31-2018, 2018.
Aroutiounian, V. M., Adamyan, A. Z., Khachaturyan, E. A., Adamyan, Z. N., Hernadi, K., Palai, Z., Nemeth, Z., Forro, L., Magrez, A., and Horvath, E.: Study of the surface-ruthenated SnO2/MWCNT nanocomposite thick-film gas sensors, Sensor. Actuat. B-Chem., 177, 308–315, https://doi.org/10.1016/j.snb.2012.10.106, 2013.
Benedet, J., Lu, D., Cizek, K., La Belle, J., and Wang, J.: Amperometric sensing of hydrogen peroxide vapor for security screening, Anal. Bioanal. Chem., 395, 371–376, https://doi.org/10.1007/s00216-009-2788-7, 2009.
Bohrer, F. I., Colesniuc, C. N., Park, J., Schuller, I. K., Kummel, A. C., and Trogler, W. C.: Selective detection of vapor phase hydrogen peroxide with phthalocyanine chemiresistors, J. Am. Chem. Soc., 130, 3712–3713, https://doi.org/10.1021/ja710324f, 2008.
Chen, S., Yuan, R., Chai, Y., and Hu, F.: Electrochemical sensing of hydrogen peroxide using metal nanoparticles: a review, Microchim. Acta, 180, 15–32, https://doi.org/10.1007/s00604-012-0904-4, 2013.
Chen, W., Cai, S., Ren, Q.-Q., Wen, W., and Zhao, Y.-D.: Recent advances in electrochemical sensing for hydrogen peroxide: a review, Analyst, 137, 49–58, https://doi.org/10.1039/c1an15738h, 2012.
Chen, X., Wu, G., Cai, Z., Oyama, M., and Chen, X.: Advances in enzime-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid, Microchim. Acta, 181, 689–705, https://doi.org/10.1007/s00604-013-1098-0, 2014.
Corveleyn, S., Vandenbossche, G. M. R., and Remon, J. P.: Near-infrared (NIR) monitoring of H2O2 vapor concentration during vapor hydrogen peroxide (VHP) sterilization, Pharm. Res., 14, 294–298, 1997.
Ensafi, A. A., Rezaloo, F., and Rezaei, B.: Electrochemical sensor based on porous silicon/silver nanocomposite for the determination of hydrogen peroxide, Sensor. Actuat. B-Chem., 231, 239–244, https://doi.org/10.1016/j.snb.2016.03.018, 2016.
Hsu, C.-C., Lo, Y.-R., Lin, Y.-C., Shi, Y.-C., and Li, P.-L.: A spectrometric method for hydrogen peroxide concentration measurement with a reusable and cost-efficient sensor, Sensors, 15, 25716–25729, https://doi.org/10.3390/s151025716, 2015.
Kačer, P., Švrček, J., Syslová, K., Václavík, J., Pavlík, D., Červený, J., and Kuzma, M.: Vapor phase hydrogen peroxide – method for decontamination of surfaces and working areas from organic pollutants, in: Organic pollutants ten years after the Stockholm Convention – environmental and analytical update, edited by: Puzyn, T., InTech, 399–430, https://doi.org/10.5772/33451, 2012.
Lin, C.-Y. and Chang, C.-T.: Iron oxide nanorods array in electrochemical detection of H2O2, Sensor. Actuat. B-Chem., 220, 695–704, https://doi.org/10.1016/j.snb.2015.06.022, 2015.
Mills, A., Grosshans, P., and Snadden, E.: Hydrogen peroxide vapour indicator, Sensor. Actuat. B-Chem., 136, 458–463, https://doi.org/10.5772/33451, 2009.
Oberländer, J., Kirchner, P., Boyen, H.-G., and Schöning, M. J.: Detection of hydrogen peroxide vapor by use of mandanese(IV) oxide as catalyst for calorimetric gas sensors, Phys. Status Solidi A, 211, 1372–1376, https://doi.org/10.1002/pssa.201330359, 2014.
Pogacean, F., Socaci, C., Pruneanu, S., Biris, A. R., Coros, M., Magerusan, L., Katona, G., Turcu, R., and Botodi, G.: Graphene based nanomaterials as chemical sensors for hydrogen peroxide – A comparison study of their intrinsic peroxidase catalytic behavior, Sensor. Actuat. B-Chem., 213, 474–483, https://doi.org/10.1016/j.snb.2015.02.124, 2015.
Puganova, E. A. and Karyakin, A. A.: New materials based on nanostructured Prussian blue for development of hydrogen peroxide sensors, Sensor. Actuat. B-Chem., 109, 167–170, https://doi.org/10.1016/j.snb.2005.03.094, 2005.
Rahim, H. A., Morat, B. C., and Rahim, R. A.: Non-invasive evaluation of hydrogen peroxide concentrations in a drinking bottle by near-infrared spectrometry, Sensors & Transducers, 131, 83–90, 2011.
Šljukič, B., Banks, C. E., and Compton, R. G.: Iron oxide particles are the active sites for hydrogen peroxide sensing at multiwalled carbon nanotube modified electrodes, Nano Lett., 6, 1556–1558, https://doi.org/10.1021/nl060366v, 2006.
Sun, J., Li, C., Qi, Y., Guo, S., and Liang, X.: Optimizing colorimetric assay based on V2O5 nanozymes for sensitive detection of H2O2 and glucose, Sensors, 16, 584–595, https://doi.org/10.3390/s16040584, 2016.
Taizo, I., Sinichi, A., and Kawamura, K.: Application of a newly developed hydrogen peroxide vapor phase sensor to HPV sterilizer, PDA J. Pharm. Sci. Tech., 52, 13–18, 1998.
Wu, Z.-L., Li, C.-K., Yu, J.-G., and Chen, X.-Q.: MnO2/reduced graphene oxide nanoribbons: facile hydrothermal preparation and their application in amperometric detection of hydrogen peroxide, Sensor. Actuat. B-Chem., 239, 544–552, https://doi.org/10.1016/j.snb.2016.08.062, 2017.
Yang, X., Ouyang, Y., Wu, F., Hu, Y., Ji, Y., and Wu, Z.: Size controllable preparation of gold nanoparticles loading on grapheme sheets@cerium oxide nanocomposites modified gold electrode for nonenzymatic hydrogen peroxide detection, Sensor. Actuat. B-Chem., 238, 40–47, https://doi.org/10.1016/j.snb.2016.07.016, 2017.
Zheng, J. Y., Yan, Y., Wang, X., Shi, W., Ma, H., Zhao, Y. S., and Yao, J.: Hydrogen peroxide vapor sensing with organic core/sheath nanowire optical waveguides, Adv. Mater., 24, OP194–OP199, https://doi.org/10.1002/adma.201200867, 2012.