the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Experimental setup to study poisoning effects of different materials on chemical sensors used in E-nose systems
Beatrice Julia Lotesoriere
Stefano Robbiani
Ana Maria Tischer
Lucia Corrà
Emanuele Zanni
Anna Gianfranceschi
Lucia Giuffrida
Raffaele Dellacà
Laura Capelli
Electronic nose (E-nose) technology relies on partially specific electronic chemical sensor arrays with an appropriate pattern recognition system housed in dedicated chambers and coupled with sampling systems to analyze simple or complex odors. An optimized design and dimensioning of the sensor chamber and sampling system can significantly improve sensor responses. In this context, the design of E-nose sampling systems has recently benefited from emerging technologies such as additive manufacturing (i.e., 3D printing) and innovative materials. While new materials can enable new functionalities in sensor housing construction, their potential gaseous emission can compromise sensor performance over time. More broadly, materials used in E-nose components and in the sampling environment can release volatile organic compounds (VOCs) that can irreversibly adsorb onto sensor surfaces, interfering with sensor functionality, also known as “poisoning”. This study aims to develop an initial experimental methodology and a dedicated setup to assess the potential poisoning effect of materials commonly used in E-nose components or typically found in the sampling environment – PEEK (polyetheretherketone), biocompatible resin and silicone – on gas sensor performance. For this purpose, two widely used commercial metal oxide semiconductor (SMOX) sensors (TGS2610 and TGS2611) were exposed to these materials in an accelerated poisoning test over 2 weeks. The results indicated that silicone and biocompatible 3D-printed resin, even after thermal pre-treatment, significantly altered sensor responses, whereas PEEK did not show any effect on sensor sensitivity over the test duration.
- Article
(1474 KB) - Full-text XML
- BibTeX
- EndNote
The E-nose is a device capable of mimicking the olfactory system (Alfieri et al., 2024; Robbiani et al., 2023). Since the introduction of the concept of E-nose in the 1980s, this technology has been progressively subjected to improvements in the ability to detect and analyze complex odors (Rabehi et al., 2024). The improved functionalities of E-nose devices have enabled the use of this technology as a tool in various fields (Furizal et al., 2023). For instance, the E-nose has emerged as a low-cost and user-friendly device contributing to food quality assistance, origin tracing, process optimization and waste reduction (Rabehi et al., 2024). In the healthcare system, the E-nose has been investigated as a less invasive tool for disease diagnosis and monitoring (Abideen et al., 2024). The volatile organic compounds (VOCs) generated by specific metabolic pathways were indeed associated with different medical conditions (e.g., diabetes, lung infections, certain type of cancers) (Rabehi et al., 2024). Furthermore, E-nose has found a wide range of applications in the environmental sector, which is a broad term comprising measurement of parameters related to air or water quality and process control (Capelli et al., 2014). When developing an E-nose for a specific application, several aspects need to be taken into account starting from the sensor selection, which is of outmost importance as fewer and application-suited sensors can significantly cut the cost of the final device and improve its portability (Cheng et al., 2021). In some instances, such as developing smart home appliances containing E-nose and/or generic sensors, the selection of suitable sensors is a necessary step as it can determine the whole device profitability (Lui, 2009). Additional key aspects to be considered when building an E-nose are the sampling lines and the measurement chamber design. In particular, Falcitelli et al. (2002) showed that the geometry of the sensing platform chamber, which houses the sensors, greatly determines sample concentration and its distribution on sensors' sensitive layer (Falcitelli et al., 2002). As a rapid and cost-effective way to produce components with complex geometry, 3D printing has been considered in the production of sensing platform chambers (Wojnowski et al., 2020) and sampling equipment due to the advancement in the technical performances of printers and the increased availability of low-cost printing devices. Especially in the biomedical field, 3D printing is increasingly being considered to manufacture medical parts and equipment (Pham et al., 2022). As an example, in the field of breath analysis, a mouthpiece adapter has been designed with 3D printers as an alternative to overcome some of the drawbacks associated with the currently used silicon face masks (Pham et al., 2023). As breath analyzer devices are designed for disease diagnosis via the detection of incredibly low traces of VOCs, the commercial resins (e.g., Surgical Guide; Formlabs Inc., Somerville, MA, USA) used for 3D printing were investigated by Pham et al. (2023) as a possible source of emission and/or uptake of confounding VOCs (Pham et al., 2023). In the same work, a reduced VOC emission was evidenced when the commercial resin was subjected to pre-treatments such as baking or autoclaving (Pham et al., 2023). In general, the materials used for the design of sensor chambers and sampling lines can alter gas sensor measurement in several ways. Microporous materials can act as VOC traps and thus temporarily hindering the target molecules (i.e., material adsorption) (Falcitelli et al., 2002). Furthermore, materials may emit VOCs that can possibly interfere with the detection of target molecules by hindering their interaction with the sensor's sensitive layer (Falcitelli et al., 2002). This blocking effect can be just temporary or reversible (e.g., in the case of the adsorption of less volatile molecules on the sensitive layer they may be “burned off” by heating up the sensor to elevated temperatures), but in some cases the interaction may be irreversible and lead to a continuous decrease in sensor sensitivity. This irreversible effect is commonly referred to as “poisoning” (Schultealbert et al., 2020). As an example, siloxanes are known to irreversibly interact with the sensitive layer and change the properties of gas sensors in terms of response time, sensitivity and selectivity (Schultealbert et al., 2020). The decomposition of siloxanes on the hot sensor surface leads to the generation of non-volatile reaction products that cause a reduction in catalytic activity known as poisoning in the metal oxide semiconductor (SMOX) community (Schultealbert et al., 2020). These VOCs are produced by silicone, which is a material widely used in several fields, ranging from sealings, face masks, and home appliances to bake ware (Barber et al., 2009). Therefore, as an example, when designing an E-nose to be included in a household appliance, the environment where the sample is sourced should also be considered to be a possible element that affects the measurement. In summary, among the various factors to consider when developing an E-nose, identifying interfering VOCs and their sources was found to be key in successful studies (Falcitelli et al., 2002). As shown in Fig. 1, interfering VOCs and poisoning agents hindering the detection of the VOCs of interest and blocking their recognition from the sensors' sensitive layer can be found at all stages in the E-nose sampling process: (1) the environmental interferences, (2) the sampling line and (3) the sensor chamber material.
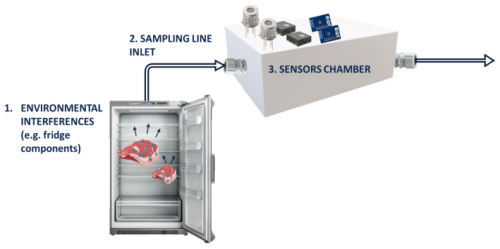
Figure 1E-nose building blocks as a source of confounding VOCs: 1. environmental interferences, 2. sampling line inlet and 3. sensor chamber.
Despite the importance of investigating interferences on E-nose sensors, studies specifically investigating the release of VOCs and quantifying the potential effect of materials on different SMOX sensors are lacking in the scientific literature (Falcitelli et al., 2002; Magnano et al., 2024; Pham et al., 2023). Moreover, in the few studies investigating the impact of VOC emission on SMOX sensor poisoning, an appropriate methodology for its evaluation is missing. For example, in the study carried out by Rohde et al. (2023) only 12 h of contact of a SMOX sensor with resin was investigated prior to usage in the final application; this short time may be not enough to evidence a change in sensor performance (Rohde et al., 2023). In this context, the aim of this work is to propose an experimental setup and a methodology to evaluate the poisoning effect of different materials, which can be of interest for designing E-nose components (e.g., sensor chamber or sampling systems) or be present as environmental interferents on a set of selected SMOX sensors.
2.1 Materials
The use of additive manufacturing and innovative materials has expanded beyond E-nose chamber and sampling inlet development to various applications, such as healthcare and the automotive industry. As a result, studying VOC emissions from 3D-printed and innovative materials, along with their potential poisoning effects on sensors, has become crucial for E-nose design (Pham et al., 2023). Different materials were selected based on an in-depth study of the literature to evaluate their poisoning effect on two SMOX sensor types commonly used in E-nose applications, i.e., the TGS2610 C-00 and the TGS2611 C-00 (Figaro Inc., Osaka, Japan) (Paradowska-Stolarz et al., 2023; Pham et al., 2023; Verma et al., 2021). Silicone R Pro 30 (Reschimica Srl, Florence, Italy) was tested due to its known poisoning effect as a sort of positive control to induce the expected changes in the sensor performance (Schultealbert et al., 2020), Moreover, a biocompatible resin (Biomed Clear, Formlabs, Massachusetts, USA), based on an acrylate–urethane polymer, was selected as it has been increasingly employed in the production of 3D-printed health devices (Paradowska-Stolarz et al., 2023). The same resin was also tested with a pre-treatment in distilled water for 2 h at 100 °C to investigate the effectiveness of applying a possible way to remove the presence of VOCs on the surface (Pham et al., 2023). Polyetheretherketone (Ensinger, Milan, Italy) was chosen due to its extensive usage in the biomedical, automotive and aerospace industry. PEEK indeed shows outstanding properties such as high mechanical strength, better thermal stability, higher chemical resistance, good wear resistance and anticorrosive nature (Verma et al., 2021).
2.2 Experimental setup
The experimental setup for testing the sensors before and after the “poisoning” period (as described in the next section) comprised five couples of the SMOX sensors mentioned before (i.e., TGS2610 C-00 and TGS2611 C-00) and one temperature and humidity sensor (i.e., a sensor SHT 40 by Sensirion, Zurich, Switzerland), which were installed in an airtight chamber. The chamber, which has a volume of 2.6 L, is constructed from glass and polytetrafluoroethylene (Teflon), both of which are well known for their inert properties. The sensor chamber was further equipped with a fan, which had the function of improving gas homogeneity within the case. A scheme of this experimental setup is shown in Fig. 2.
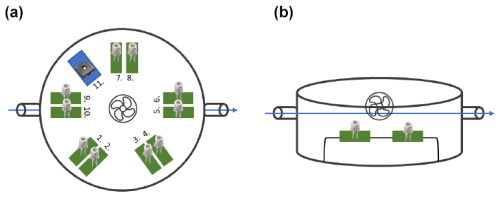
Figure 2Chamber schematization from two different perspectives. Panel (a) shows the perspective from the top, showing the 11 sensors selected for this study. Sensors 1, 3, 5, 7 and 9 are TGS2610, sensors 2, 4, 6, 8 and 10 are TGS2611, and sensor 11 is SHT40 for temperature and humidity. Panel (b) displays the lateral perspective of the chamber, highlighting the relative positions of the sensors with respect to the airflow and the fan.
Sensor performance was evaluated using this experimental setup before and after subjecting them to the different materials by measuring sensor responses with different calibrants. The sensors were kept connected and switched on for the duration of the whole experimental part in order to have information on the sensor behavior over time. The experimental part comprised the following steps.
Firstly, the sensors were conditioned with ambient air for 7 d following the conditioning recommendation guidelines on the sensor datasheets as reported in Appendix A1.
Subsequently, gas sensor performance was evaluated by measuring the resistance towards different known calibrants: butanol 20 ppm, isobutylene 20 ppm, 4-heptanone 20 ppm and methane 750 ppm.
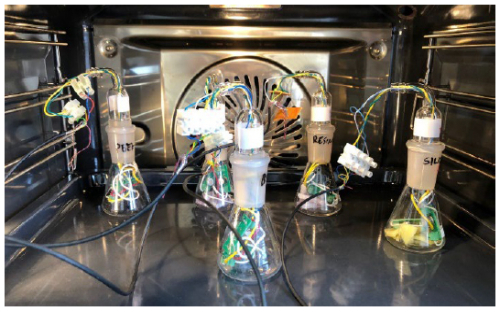
After evaluating their performances, accelerated poisoning was carried out. The accelerated poisoning was carried out as follows: sensors were placed in pairs (i.e., one TGS2610 and one TGS2611) in five flasks of 50 mL StonyLab®. One flask, containing only the two sensors, served as the blank condition (no exposure to poisoning agents), while the other four flasks each contained a different material, with an equivalent surface area of 11 cm2. To accelerate the VOC release and thus sensitive layer interaction with the released VOCs (accelerated poisoning), the flasks containing a pair of sensors each were placed in a ventilated oven (AEG BSK798280B) at 80 °C for 15 d (Fig. 3).
Following the accelerated poisoning in the oven, sensors were placed back in the chamber used for the tests of step 2 (Fig. 2), and the same tests as before poisoning were repeated at the same controlled conditions (i.e., 40 % RH, 22 °C).
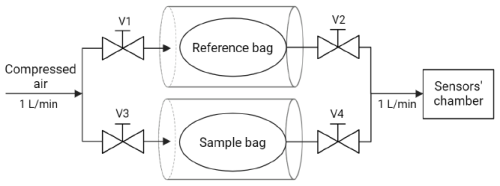
Regarding the preparation of the calibrant samples, the liquid calibrant 4-heptanone (Sigma-Aldrich., St. Louis, Missouri, USA) was prepared at 20 ppm by injecting it with an NE-300 Just InfusionTM Syringe Pump (New Era Pump Systems, Inc., Toledo St, Farmingdale, USA) continuously in a volatile stream of filtered air with a stable and defined flow rate obtained with Alicat™ portable calibration unit mass flowmeters (Sartore et al., 2022). The 4-heptanone was then stored in NalophanTM bags for 3 h to equilibrate with the environment of the room at T=22 °C and RH = 40 %. Isobutilene, butanol and methane were gaseous calibrants stored in gas cylinders (SAPIO, Monza, Italy), and the final concentration was reached by diluting with filtered air using a 1 L Super Syringe (Hamilton, Germany). Before starting the analysis, the concentrations of 4-heptanone, isobutylene and butanol were checked using a TIGER photoionization detector (PID) (ION Science Limited, UK), whereas methane concentration was measured with a flame ionization detector (FID) (Gastec MK 5, Crowcon Detection Instruments Limited). All calibrant samples were prepared to have the same relative humidity (i.e., 40 %), and the room with the experimental setup and the samples was conditioned to keep temperature and humidity constant at T=22 °C and RH = 40 % in order to minimize effects on the sensor responses due to the variation of such environmental parameters. Calibrant samples were analyzed alternately with reference air. Reference air is compressed air filtered in order to remove any impurities and then humidified at the same level as the samples (i.e., RH = 40 %) and stored in a NalophanTM bag. The humidity and temperature sensor SHT 40 was added to the experimental setup to double-check that humidity and temperature were effectively constant across the different tests. Since the chamber depicted in Fig. 2 is not airtight, we needed to design a dedicated sample delivery system in order to press out the sample from the NalophanTM bag into the sensor chamber. The dedicated sample delivery system (Fig. 4) enables the alternate injection into the sensor chamber of the target gas sample and reference air, avoiding any contact of the target gas samples with mechanical parts or pumps, which may be a possible source of undesired VOC emission. The system consists of two sealed cylinders (Fig. 4): one cylinder (reference bag in Fig. 4) contained the reference air and the other the target calibrant gas (sample bag in Fig. 4), both stored in NalophanTM bags. Compressed air at 1 L min−1 is injected to pressurize the cylinder by opening valve V1 and valve V3, allowing for the release of either the calibrant gas sample or the reference air from the NalophanTM bag into the sensor chamber. Each test lasted 45 min and consisted of around 15 min of reference air (determined by the volume of the reference bag), around 15 min of sample (determined by the volume of the sample bag) and around 15 min of reference air again by substituting the reference bag previously used. This relatively simple system has the main advantage of avoiding any contact of the gas to be analyzed with possible interfering materials other than Teflon, NalophanTM or glass, which are known to be inert and belong to the materials allowed for olfactometric sampling (according to EN 13725:2022), thus enabling us to exclude any source of interference other than the material under examination.
The temperature and duration of the sensor exposure of 80 °C and 15 d were chosen as they were considered sufficient to explore the tested materials' potential to alter the sensors responses. Indeed, the durability test reported in the datasheet of the TGS2611 C-00 sensor, which in turn follows Item 5.3.13 of the European Standard EN 50194, foresees an exposure of the sensor to HDMS (hexamethyl disiloxane) for just 40 min. The conditions chosen in this study are therefore more conservative. This choice is also supported by other literature studies (e.g., Pham et al., 2023), where a 12 h treatment at 78 °C showed a reduction of emissions from the material from 595 to 225 ppbv, thus indicating that at this temperature most of the VOCs are released by the materials within a limited time.
2.3 Evaluation of sensor responses before and after poisoning
As described in the previous paragraph, the sensor response curves were determined by acquiring the raw value of the sensor resistance during the analysis of the reference air followed by the gaseous calibrant and then reference air again. The raw resistance values were normalized as .
As will be shown in the next section, the normalized resistances were plotted in order to enable comparison of the sensor response curves before and after poisoning. Moreover, sensitivity (%) to each calibrant was calculated for each sensor included in the tests in order to evaluate if and how the sensitivity to the target gases was modified after exposition to the different materials. Sensitivity (%) was calculated according to Nadargi et al. (2023) as , where R is the last resistance point recorded at the end of the analysis when steady state is reached and R0 is the first value acquired.
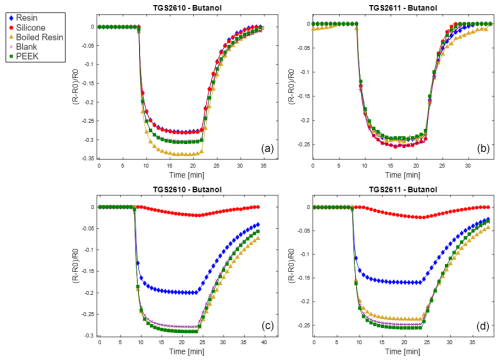
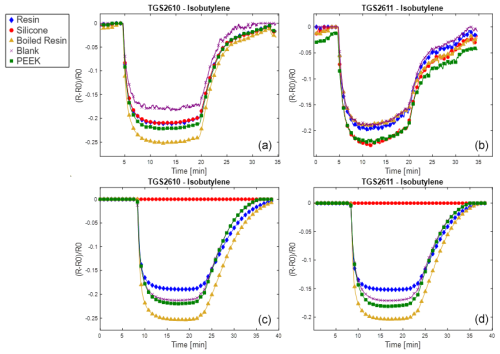
Results are reported here for butanol and methane, which were the most significant compounds to analyze the poisoning effect of the different materials tested. Analogous results for the other two calibrants (i.e., 4-heptanone and isobutylene) are provided in the Appendix (Figs. A1 and A2). The normalized sensor response curves obtained before and after poisoning are displayed in Figs. 5 and 6. Each figure reports the curves for the two sensor types (i.e., TGS2610 and TGS2611) towards the two calibrants before (in the upper part of the figure) and after (in the lower part of the figure) poisoning. In order to enable a more quantitative comparison, sensitivities calculated as explained in Sect. 2.3 are reported as bar plots in Figs. 7 and 8 for TGS2610 and 2611, respectively. It is evident in all the figures that the curves relevant to the sensors exposed to silicone (red line) show a response that is almost zeroed after the poisoning tests, which is also confirmed by the sensitivity dropping below 5 % in all cases. A reduced response and sensitivity are also observed for the sensors exposed to the resin: both TGS2610 and 2611 show a decrease in sensitivity of more than −5 % to butanol and more than −10 % for methane. With regards to PEEK and blank lines (green and purple lines, respectively) the responses are, as expected, substantially unvaried before and after. Regarding the sensors exposed to boiled resin (yellow lines in Figs. 5 and 6), they exhibit an intermediate response compared to those exposed to resin and silicone. In particular, after the poisoning tests, a 5 % decrease in sensitivity is observed for methane, whereas no such decrease is seen for butanol.
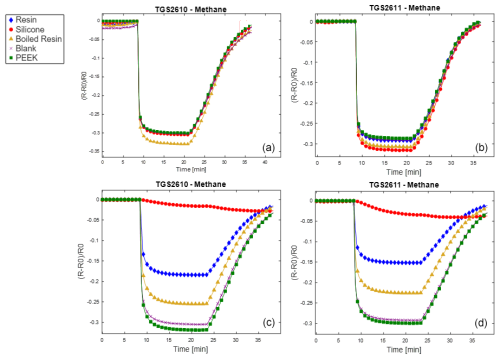
Figure 6Normalized responses to methane for the two sensor types (i.e., TGS2610 and TGS2611) before (a, b) and after (c, d) poisoning. The legend entries specify which material was used to poison the sensor.
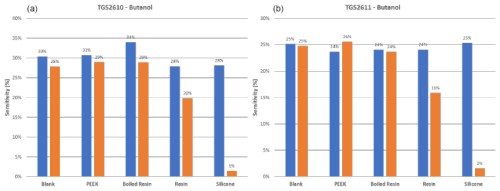
Figure 7Sensitivity (in %) to butanol for TGS2610 (a) and TGS2611 (b) sensors before and after poisoning. Data were calculated using the formula explained in Sect. 2.3 (“Materials and methods”).
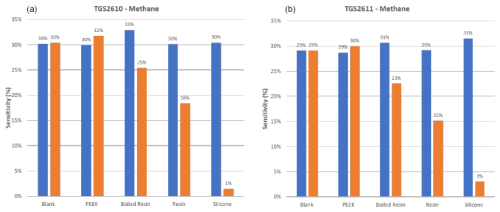
Figure 8Sensitivity (in %) to methane for TGS2610 (a) and TGS2611 (b) sensors before and after poisoning. Data were calculated using the formula explained in Sect. 2.3 (“Materials and methods”).
Since the functioning principle of SMOX sensors involves direct chemical interaction between the sensitive layer and the environment, they are prone to poisoning by irreversible adsorption of unwanted molecules (Chai et al., 2022). Silicone and the volatile siloxane emitted, as already widely reported, are known to change the SMOX sensor properties (Schüler et al., 2015). The volatile siloxanes can generate non-volatile aggregates on reactive surfaces and its catalysts; this interaction has been shown to change the sensor properties, such as sensitivity and selectivity (Schüler et al., 2015). As previously described, the poisoning with silicone drastically changed the sensor properties. As a consequence, the response is almost zeroed (flat red line in Figs. 5 and 6) and the sensitivity dropped to below 5 %, making the sensors useless. As previously stated, resin (blue line in Figs. 5 and 6) also changed the sensor responses. VOC emission from 3D-printed resin (Surgical Guide; Formlabs Inc., Somerville, MA, USA) was extensively investigated by Pham et al. (2023). As proven in their study, the most abundant VOCs emitted from the resin were propylene glycol, followed by acetaldehyde, propionic acid and mesitaldehyde (Pham et al., 2023). The SMOX sensitive layer, due to the high surface activity, may irreversibly interact with the former molecules, leading to a decline of the long-term stability of semiconductors (i.e., poisoning effect) (Chai et al., 2022). Different types of treatments including a hydrothermal step are generally performed on materials intended for biomedical purposes (Pham et al., 2022). These treatments not only guarantee sterility but also result in marked reductions in the overall VOC emissions (Pham et al., 2023). The boiled resin normalized response (yellow line) changed the sensor responses only when tested with methane, indicating that a resin pre-treatment may significantly reduce the material's excessive presence of VOCs. However, the significant reduction of the sensor responses observed after the accelerated poisoning experiment in the oven may indicate that this type of pre-treatment is not sufficient to make the material compatible with use in E-nose applications. Other literature studies are proposing different pre-treatments of the resin (i.e., UV curing followed by vacuum baking at 50 °C for 24 h) prior to developing the E-nose for the final application (Li et al., 2023). Even though these pre-treatments are proven to reduce off-gassing during use, their effect on the long-term poisoning of SMOX sensors was not investigated (Li et al., 2023). According to our results, the only material that did not produce an alteration of the sensor responses over time is PEEK. As expected, responses of the sensors following PEEK exposure overlapped the blank (i.e., no materials) responses (green and purple lines, respectively), proving that this material has no poisoning effect on the studied SMOX sensors. PEEK indeed represents an extremely interesting material for designing E-nose sensor chambers as well as sampling systems, suitable for uses in different sectors, including biomedical applications (Paradowska-Stolarz et al., 2023). However, PEEK is not suitable for 3D printing, making the manufacturing of PEEK items more complex and expensive compared to the use of 3D-printable materials (Verma et al., 2021). Moreover, its behavior is not known when in contact with complex gases as it can act as adsorbent material (Pham et al., 2023). Future studies should investigate the possible release of VOCs of PEEK after having been exposed to odorant mixtures.
This study had the goal of proposing an experimental methodology to assess the compatibility of different materials with SMOX operation, with the purpose of evaluating suitability for the manufacturing of E-nose sampling systems and sensor chambers, but also to investigate possible interferences with the environment where the gas to be analyzed is taken. The proposed methodology included the design of an experimental setup that enabled the evaluation of the poisoning effect as a change in the performance of SMOX sensors excluding all the other possible confounding factors (e.g., electronic board, mechanical parts or pumps). The change in performance was evaluated by measuring sensor response before and after accelerated poisoning of different materials (PEEK, resin and silicone) and pre-treatment (boiling of resin). Results showed silicone to have a detrimental effect on SMOX sensors. Thus, silicone should be carefully avoided in all surrounding environments of E-nose components in order to ensure that the gas to be analyzed does not get in contact with this poisoning material. Excluding silicone may be difficult in some applications, considering that silicone is commonly used in the realization of sealings or flexible tubing. Because of this poisoning effect of siloxanes, recently, some siloxane-resistant SMOX sensors have been introduced into the market, such as the SGP40 (Sensirion AG, Switzerland), which is equipped with a protective filter (Appendix A1). Additionally, it is claimed that ZMOD4410 and ZMOD4510 (Renesas, Germany) are siloxane-resistant (Appendix A1). However, despite their resistance to siloxanes, their performance robustness when in contact with the VOC emission from other materials such as 3D-printable resins is not reported. Future studies should therefore extend the analysis to these types of sensors to verify whether they are also suitable to be used in combination with such biocompatible resin. As a matter of fact, the preliminary results obtained in this study show that 3D-printable resin (Biomed Clear, Formlabs, Massachusetts, USA), which is commonly used in biomedical devices because of its biocompatibility and ease of processability, is not compatible for the development of E-nose components when TGS sensors are used. The applied pre-treatment (i.e., boiling at 100 °C for 2 h) reduced the poisoning effect of the resin on the sensors but did not eliminate it, thus suggesting that future studies should investigate the possibility of applying more severe conditions for resin thermal pre-treatment in order to make it suitable for use in E-nose applications, such as heating at 120 °C using a steamer or dry-heating in an oven at 120 °C for 2 h (Pham et al., 2023). The results obtained also indicated PEEK as a material that does not produce poisoning effects on the investigated sensors. However, PEEK's higher melting temperatures make it unsuitable for 3D printing (Verma et al., 2021). Additionally, its higher cost hampers its usage for the building of E-nose cases in low-margin devices such as household appliances. Further investigation of PEEK's VOC adsorption following exposure to complex odorant mixtures is needed. Future tests are foreseen to confirm these preliminary results with the calculation of performance parameters such as repeatability, sensitivity and response time. Moreover, this study will be extended with the purpose of investigating other sensors and other materials, such as 3D-printable PETG (polyethylene terephthalate glycol-modified) or PLA (polylactic acid), which are also commonly used for the realization of E-nose components (Cheng et al., 2020).
-
TGS2610-C00: Figaro Engineering Inc.: TGS2610-C00 sensor for LP Gas, https://www.figarosensor.com/product/entry/tgs2610-c00.html (last access: 30 May 2025).
-
TGS2611-C00: Figaro Engineering Inc.: TGS2611-C00 sensor for methane, https://www.figarosensor.com/product/entry/tgs2611-c00.html (last access: 30 May 2025).
-
SGP40: Sensirion Holding AG: SGP40 VOC sensor for indoor air quality applications, https://sensirion.com/products/catalog/SGP40 (last access: 30 May 2025).
-
ZMOD4410: Renesas Electronics: ZMOD4410 firmware configurable indoor air quality (IAQ) sensor with embedded artificial intelligence (AI), https://www.renesas.com/en/products/general-parts/zmod4410-firmware-configurable-indoor-air-quality-iaq (last access: 30 May 2025).
-
ZMOD4510: Renesas Electronics: ZMOD4510 gas sensor for O3 and NO2, https://www.renesas.com/en/products/general-parts/zmod4510-gas-sensor-o3-and-no2 (last access: 30 May 2025).
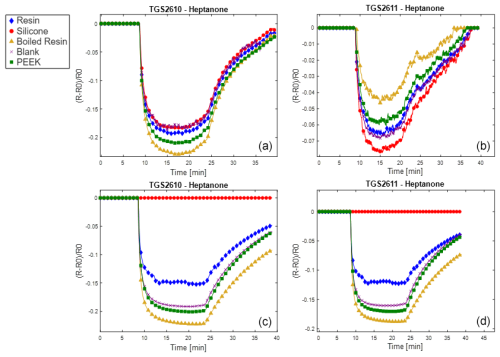
Figure A1Normalized responses to 4-heptanone for the two sensor types (i.e., TGS2610 and TGS2611) before (a, b) and after (c, d) poisoning. The legend entries specify which material was used to poison the sensor.
All the measurement data and codes are not publicly available but can be made available upon request to the corresponding author.
BJL, SR, RD and LCa worked on the conceptualization and designed the experiments, while AMT, LCo, LG, EZ and AA carried them out. AMT analyzed the obtained results. LCo prepared the paper with contributions from all co-authors. LCa and RD contributed with substantial revisions.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This article is part of the special issue “Eurosensors 2024”. It is a result of the EUROSENSORS XXXVI, Debrecen, Hungary, 1–4 September 2024.
This paper was edited by Gabor Battistig and reviewed by three anonymous referees.
Abideen, Z. U., Arifeen, W. U., and Bandara, Y. M. N. D. Y.: Emerging trends in metal oxide-based electronic noses for healthcare applications: a review, Nanoscale, 16, 9259–9283, https://doi.org/10.1039/D4NR00073K, 2024.
Alfieri, G., Modesti, M., Riggi, R., and Bellincontro, A.: Recent Advances and Future Perspectives in the E-Nose Technologies Addressed to the Wine Industry, Sensors, 24, 2293, https://doi.org/10.3390/s24072293, 2024.
Barber, N., Broz, C., and Boyce, J.: Silicone adjustable bundling devices: do they meet manufacturer's claims during cooking and cleaning?, J. Foodserv., 20, 63–70, https://doi.org/10.1111/j.1748-0159.2008.00126.x, 2009.
Capelli, L., Sironi, S., and Del Rosso, R.: Electronic Noses for Environmental Monitoring Applications, Sensors, 14, 19979–20007, https://doi.org/10.3390/s141119979, 2014.
Chai, H., Zheng, Z., Liu, K., Xu, J., Wu, K., Luo, Y., Liao, H., Debliquy, M., and Zhang, C.: Stability of Metal Oxide Semiconductor Gas Sensors: A Review, IEEE Sens. J., 22, 5470–5481, https://doi.org/10.1109/JSEN.2022.3148264, 2022.
Cheng, L., Liu, Y.-B., and Meng, Q.-H.: A Novel E-Nose Chamber Design for VOCs Detection in Automobiles, in: 2020 39th Chinese Control Conference (CCC), 27–29 July 2020, Shenyang, China, 6055–6060, https://doi.org/10.23919/CCC50068.2020.9189213, 2020.
Cheng, L., Meng, Q.-H., Lilienthal, A. J., and Qi, P.-F.: Development of compact electronic noses: a review, Meas. Sci. Technol., 32, 062002, https://doi.org/10.1088/1361-6501/abef3b, 2021.
Falcitelli, M., Benassi, A., Di Francesco, F., Domenici, C., Marano, L., and Pioggia, G.: Fluid dynamic simulation of a measurement chamber for electronic noses, Sens. Actuat. B, 85, 166–174, https://doi.org/10.1016/S0925-4005(02)00071-0, 2002.
Furizal, F., Ma'arif, A., Firdaus, A. A., and Rahmaniar, W.: Future Potential of E-Nose Technology: A Review, Int. J. Robot. Control Syst., 3, 449–469, https://doi.org/10.31763/ijrcs.v3i3.1091, 2023.
Li, J., Hannon, A., Yu, G., Idziak, L. A., Sahasrabhojanee, A., Govindarajan, P., Maldonado, Y. A., Ngo, K., Abdou, J. P., Mai, N., and Ricco, A. J.: Electronic Nose Development and Preliminary Human Breath Testing for Rapid, Non-Invasive COVID-19 Detection, ACS Sens., 8, 2309–2318, https://doi.org/10.1021/acssensors.3c00367, 2023.
Lui, T. J.: Automation in Home Appliances, in: Springer Handbook of Automation, Springer, Berlin, Heidelberg, 1469–1483, https://doi.org/10.1007/978-3-540-78831-7_83, 2009.
Magnano, M. C., Ahmed, W., Wang, R., Bergant Marušič, M., Fowler, S. J., and White, I. R.: Exhaled volatile organic compounds and respiratory disease: Recent progress and future outlook, Trends Anal. Chem., 176, 117739, https://doi.org/10.1016/j.trac.2024.117739, 2024.
Nadargi, D. Y., Umar, A., Nadargi, J. D., Lokare, S. A., Akbar, S., Mulla, I. S., Suryavanshi, S. S., Bhandari, N. L., and Chaskar, M. G.: Gas sensors and factors influencing sensing mechanism with a special focus on MOS sensors, J. Mater. Sci., 58, 559–582, https://doi.org/10.1007/s10853-022-08072-0, 2023.
Paradowska-Stolarz, A. M., Wieckiewicz, M., Mikulewicz, M., Malysa, A., Dus-Ilnicka, I., Seweryn, P., Laskowska, J., Figueiredo Pollamann, M. C., Adamska, M., and Wezgowiec, J.: Comparison of the tensile modulus of three 3D-printable materials used in dentistry, Dent. Med. Probl., 60, 505–511, https://doi.org/10.17219/dmp/166070, 2023.
Pham, Y. L., Beauchamp, J., Clement, A., Wiegandt, F., and Holz, O.: 3D-printed mouthpiece adapter for sampling exhaled breath in medical applications, 3D Print Med., 8, 27, https://doi.org/10.1186/s41205-022-00150-y, 2022.
Pham, Y. L., Holz, O., and Beauchamp, J.: Emissions and uptake of volatiles by sampling components in breath analysis, J. Breath Res., 17, 037102, https://doi.org/10.1088/1752-7163/acce34, 2023.
Rabehi, A., Helal, H., Zappa, D., and Comini, E.: Advancements and Prospects of Electronic Nose in Various Applications: A Comprehensive Review, Appl. Sci., 14, 4506, https://doi.org/10.3390/app14114506, 2024.
Robbiani, S., Lotesoriere, B. J., Dellacà, R. L., and Capelli, L.: Physical Confounding Factors Affecting Gas Sensors Response: A Review on Effects and Compensation Strategies for Electronic Nose Applications, Chemosensors, 11, 514, https://doi.org/10.3390/chemosensors11100514, 2023.
Rohde, A. W., Nel, J. M., and Joubert, T.-H.: Insecticide Monitoring in Cattle Dip with an E-Nose System and Room Temperature Screen-Printed ZnO Gas Sensors, Agriculture, 13, 1483, https://doi.org/10.3390/agriculture13081483, 2023.
Sartore, L., Polvara, E., Invernizzi, M., and Sironi, S.: Determination of Air Pollutants: Application of a Low-Cost Method for Preparation of VOC Mixtures at Known Concentration, Sustainability, 14, 9149, https://doi.org/10.3390/su14159149, 2022.
Schüler, M., Sauerwald, T., and Schütze, A.: A novel approach for detecting HMDSO poisoning of metal oxide gas sensors and improving their stability by temperature cycled operation, J. Sens. Sens. Syst., 4, 305–311, https://doi.org/10.5194/jsss-4-305-2015, 2015.
Schultealbert, C., Uzun, I., Baur, T., Sauerwald, T., and Schütze, A.: Siloxane treatment of metal oxide semiconductor gas sensors in temperature-cycled operation – sensitivity and selectivity, J. Sens. Sens. Syst., 9, 283–292, https://doi.org/10.5194/jsss-9-283-2020, 2020.
Verma, S., Sharma, N., Kango, S., and Sharma, S.: Developments of PEEK (Polyetheretherketone) as a biomedical material: A focused review, Eur. Polym. J., 147, 110295, https://doi.org/10.1016/j.eurpolymj.2021.110295, 2021.
Wojnowski, W., Kalinowska, K., Gębicki, J., and Zabiegała, B.: Monitoring the BTEX Volatiles during 3D Printing with Acrylonitrile Butadiene Styrene (ABS) Using Electronic Nose and Proton Transfer Reaction Mass Spectrometry, Sensors, 20, 5531, https://doi.org/10.3390/s20195531, 2020.